In Vivo Gene Therapy Isn’t The Answer. Sickle Cell Patients Need Today’s Treatments

Jimi Olaghere, technology entrepreneur, Dad, sickle cell disease patient advocate
On his fourth birthday, Adamu couldn’t celebrate.
The boy, from a rural village in Nigeria, had a flare up of his sickle cell disease known as a pain crisis. His parents tell me the rare days when he’s full of energy and playing with his friends feel like a precious gift. This year, he was given a cruel reminder of the daily battle he faces.
I was fortunate to be one of the first patients to receive a CRISPR gene edited cell therapy that cured my sickle cell disease. Adamu isn’t so lucky. There is no such cure immediately available for him.
Adamu’s father, after reading about my participation in a gene-editing clinical trial, held onto the hope that one day this breakthrough could rewrite his son’s fate. But the reality is sobering. The closest treatment center is thousands of miles away, and the cost of treatment is prohibitive. The life expectancy for SCD patients in regions like West Africa is often below five years. I suggested hydroxyurea as a possible treatment for Adamu, only to find out that his father had never heard of it. As a relatively cheap generic therapy, it is available in parts of Africa. But even access to low-cost generics can’t be taken for granted in a place with limited healthcare resources.
Adamu’s story is not unique. In low- and middle-income countries, particularly in sub-Saharan Africa and parts of Asia, healthcare systems are under-resourced, ill-equipped, and unable to manage chronic diseases like SCD. Every year, over 300,000 infants are born with SCD, and many don’t survive past childhood without early diagnosis and treatment. An estimated 5 million people worldwide suffer from chronic sickle cell disease – the vast majority of whom live in Africa.
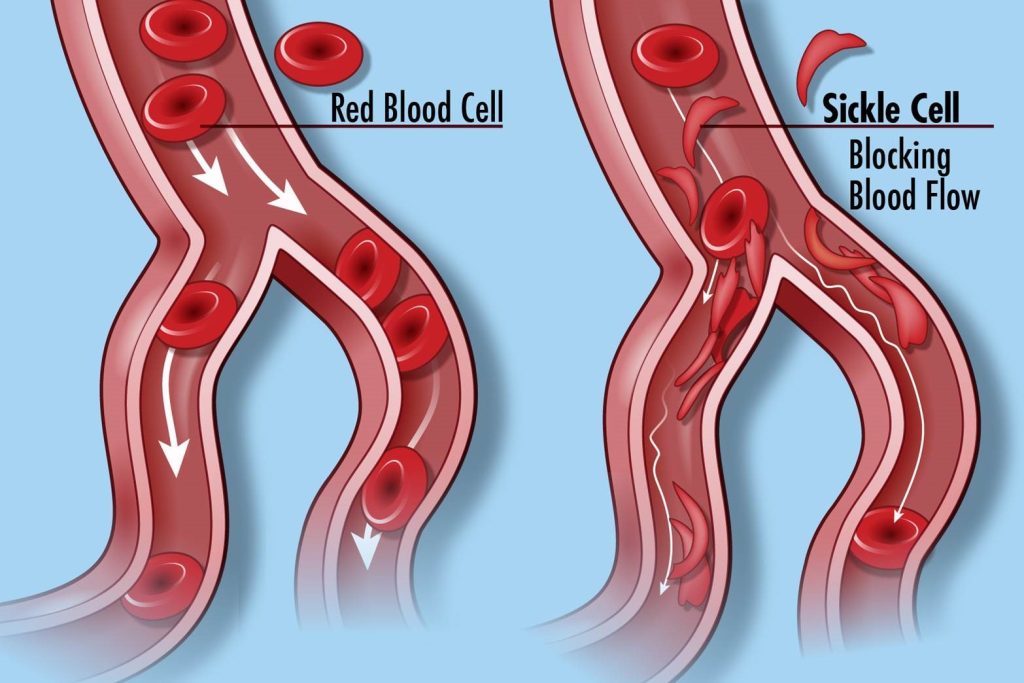
SCD is caused by a genetic mutation that results in red blood cells becoming misshapen. These cells can’t flow smoothly through blood vessels, causing painful blockages and depriving the body of oxygen. These malformed cells break down faster, leading to constant fatigue, chronic pain, organ damage, and shortened lifespans.
By National Human Genome Research Institute (NHGRI) from Bethesda, MD, USA – Sickle Cell Disease, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=52360077
There is hope on the horizon through new gene therapies that could potentially transform the lives of those with SCD. By editing genetic instructions, scientists can restore red blood cells to their healthy form, reducing the frequency of painful crises, renewing energy, and extending life. The tools to save SCD lives already exist in the form of Ex Vivo gene therapy.
The main challenge today is the cost.
To make an impact, we must reduce the cost of these existing solutions instead of waiting for future therapies that may take years to develop.
Ex Vivo gene therapy for SCD involves removing a patient’s blood stem cells, modifying them outside the body to fix the genetic problem, and then reintroducing them into the bloodstream. This approach directly addresses the disease’s root cause rather than merely managing its symptoms.
Today’s Ex Vivo Gene Therapies
The FDA recently approved two groundbreaking Ex Vivo therapies: Bluebird Bio’s Lyfgenia and Vertex Pharmaceuticals’ and CRISPR Therapeutics’ Casgevy. Lyfgenia works by adding a special anti-sickling gene into a patient’s blood-forming stem cells, helping their body make healthier red blood cells. Casgevy, using CRISPR-Cas9 technology, reprograms stem cells to produce higher levels of fetal hemoglobin by targeting and disabling a specific gene that usually suppresses it in adults.
The result is a flow of healthy red blood cells that deliver oxygen effectively, reducing painful blockages.
I am living proof of the success of Ex Vivo therapies. After 35 years with SCD, I was at the brink of a catastrophic ending, but I made a miraculous turnaround after an infusion of Casgevy in September 2020. It changed my life for the better.
These therapies offer real hope to patients like me, but they remain out of reach for the majority. The proven efficacy of Ex Vivo gene therapy makes it the most viable solution at present.
The New Scientific Frontier
In Vivo gene therapy is an exciting concept that many scientists are pursuing. It takes sickle cell treatment a step further by editing genes directly inside the patient’s body. In Vivo therapies deliver gene-editing tools into the bloodstream, where they can target and correct the genetic issue within the body.
One key advantage is they don’t require multiple blood collections, or toxic pre-conditioning treatments, like the ones I had to endure to receive an ex vivo cell therapy. They offer the theoretical possibility of short hospital stays, and one-and-done treatment.
However, In Vivo therapies are still experimental, with unknown long-term outcomes. SCD patients, especially in regions where medical care is limited, cannot afford to wait another decade or more for these treatments to become viable. Furthermore, since In Vivo gene therapy introduces genetic changes directly into the body, regulatory scrutiny is intense, adding years of research and testing before wide availability.
A significant challenge for In Vivo gene therapy is delivering gene-editing tools to the right cells — specifically, hematopoietic stem cells in the bone marrow. Ensuring accuracy and minimizing off-target effects remain technical hurdles. Additionally, In Vivo gene therapies may have unintended impacts on fertility due to potential effects on reproductive cells, a concern particularly in African contexts where family and continuity are culturally important.
Given these risks, Ex Vivo therapies, which allow for controlled editing outside the body, align better with the needs and values of SCD patients in Africa and parts of Asia.
Investing for Today and Tomorrow
One of the major advantages of Ex Vivo therapy is that the technology already exists. With streamlined protocols and increased access to treatment centers, we can begin saving lives much sooner. However, the cost remains a significant barrier today. The personalized cell therapy manufacturing process is labor-intensive and resource-heavy. Viral vectors and plasmids used in gene editing are among the most costly components.
To make Ex Vivo gene therapy more accessible, we need to reduce costs by automating and standardizing manufacturing processes. Advances in technology could make cell collection and gene editing more efficient, lowering overall costs. Reducing the cost of raw materials, such as viral vectors, will also be essential.
To reach patients in low-resource settings, investments in specialized treatment centers and training for healthcare professionals are essential. Lessons from other fields, like vaccine distribution, could provide valuable insights to build on.
The role of capitalism cannot be ignored in the conversation about curing SCD. The biotech industry’s profit motives have fueled immense investments in research and development, enabling the creation of cutting-edge therapies like gene editing. Success of ex vivo gene therapies certainly helps underwrite further scientific work on the new frontier of in vivo gene therapy. Without financial incentives, much of the progress in SCD treatment would not have been possible.
However, while the profit motive has driven innovation, it has also created barriers to accessibility. Companies can balance profit with patient needs by forming public-private partnerships and collaborations that share the financial risk of developing life-saving therapies. Additionally, relocating production and manufacturing to regions where labor is less expensive but skill levels are high can reduce operating costs.
Curing SCD in low- and middle-income countries is not just compassionate — it is strategic for high income nations as well. Reducing the global burden of SCD cuts healthcare costs worldwide by preventing chronic complications that lead to repeated hospitalizations. Healthier populations in low-income regions mean stronger economies and a more vibrant global workforce, benefiting international trade and market growth. Expanding SCD treatment globally also accelerates scientific innovation. Collaborating across borders to deliver effective therapies drives down costs and advances gene-editing techniques that ultimately benefit everyone.
Imagine what Adamu’s life could be if we save it. Today, he is a young boy whose life is defined by pain and uncertainty of when the next pain crisis might strike. But with access to gene therapy, his future could change. He could grow into adulthood full of vitality, free from SCD, able to pursue his dreams, build a career, and perhaps one day become a father himself.
Today, I am not just alive — I am thriving. I get to be a father to three beautiful, incredibly smart kids because I was given a second chance through gene therapy. Without it, I might not be here to watch them grow, teach them, and share in their accomplishments. Adamu and millions like him deserve that same future. By focusing on making Ex Vivo gene therapies more accessible, we can ensure that he, too, has the chance to build a life and pass on his strength and resilience to the next generation.

Timmerman Traverse for Sickle Forward on the summit of Kilimanjaro, Sept. 16, 2024. All 20 team members made it to the top. Jimi Olaghere, the first sickle cell disease patient to summit Africa’s highest peak, is third from the right (a patch of his teal jacket is showing).




