Biotech’s Candle Burns Bright in the Dark

Luke Timmerman, founder & editor, Timmerman Report
Earlier this week, in need of an afternoon break, I went for a 15-minute walk to the local branch of the Seattle Public Library.
With KN95 mask snugly in place, I walked in, picked up my book on hold, and walked out. Two minutes, tops. No lingering these days.
At home, I flipped open Carl Sagan’s 1996 manifesto, “The Demon-Haunted World: Science as a Candle in the Dark.”
It’s truly haunting to read 25 years later. The man was ahead of his time.
For those who want to understand the long roots of decay, I’d also recommend “Amusing Ourselves to Death: Public Discourse in the Age of Show Business” the 1985 acid commentary by Neil Postman and “Fantasyland” by Kurt Andersen in 2017.
If Sagan were around today, I’m sure he’d be in awe of what today’s scientists achieve on a daily basis. The vaccines, the diagnostics, the monoclonal antibodies, the protease inhibitors. The genomic surveillance that provides sentinel early warnings of new variants to guide policy, and let people know when to adapt their behaviors. The way in which scientific results are communicated with lightning speed around the world, elevating everyone’s game in the process.
It’s stunning.

Carl Sagan
Sagan would also surely be terrified by how things have broken down into an Internet dark age of popular pseudoscience, cynicism, ignorance, willful ignorance, institutional mistrust, bad faith arguments and apathy. To see this much preventable suffering and death happening every day as a result of these social ills would be painful.
He saw it coming. Sagan wrote:
“Science is more than a body of knowledge; it is a way of thinking. I have a foreboding of an America in my children’s or grandchildren’s time – when the United States is a service and information economy; when nearly all the key manufacturing industries have slipped away to other countries; when awesome technological powers are in the hands of a very few, and no one representing the public interest can even grasp the issues; when the people have lost the ability to set their own agendas or knowledgeably question those in authority; when, clutching our crystals and nervously consulting our horoscopes, our critical faculties in decline, unable to distinguish between what feels good and what’s true, we slide, almost without noticing, back into superstition and darkness.
The dumbing down of America is most evident in the slow decay of substantive content in the enormously influential media, the 30-second sound bites (now down to 10 seconds or less), lowest common denominator programming, credulous presentations on pseudoscience and superstition, but especially a kind of celebration of ignorance. As I write, the number one videocassette rental in America is the movie “Dumb and Dumber.” Beavis and Butt-Head remain popular (and influential) with young TV viewers. The plain lesson is that study and learning—not just of science, but of anything—are avoidable, even undesirable.”
He wrote that in 1996.
At the time, Sagan surely got pushback for sounding like a grumpy old man. Lighten up, right?
Sagan wasn’t trying to do the popular thing. He was saying something that needed to be said.
He went on to hammer home why this disconnect between science and society matters.
“We’ve arranged a global civilization in which most crucial elements – transportation, communications, and all other industries; agriculture, medicine, education, entertainment, protecting the environment; and even the key democratic institution of voting – profoundly depend on science and technology. We have also arranged things so that almost no one understands science and technology. This is a prescription for disaster. We might get away with it for a while, but sooner or later this combustible mixture of ignorance and power is going to blow up in our faces.”
That’s especially hard to read on the anniversary of the Jan. 6, 2021 insurrection. It was something a lot of people didn’t imagine, didn’t see coming.
Irritated as he was in the mid-1990s, Sagan tried to balance his frustration with hope.
As any experimentalist knows, you get out what you put in. What if we planted some different seeds?
At the end of that blistering chapter, he wrote:
“An extraterrestrial being, newly arrived on Earth—scrutinizing what we mainly present to our children in television, radio, movies, newspapers, magazines, comics, and many books—might easily conclude that we are intent on teaching them murder, rape, cruelty, superstition, credulity, and consumerism. We keep at it, and through constant repetition many of them finally get it. What kind of society could we create if, instead, we drummed into them science and a sense of hope?”
Science has the potential to make a difference against the big challenges. Pandemics, climate change, poverty, pollution and plunder of Planet Earth. But science can’t fulfill its potential against any of these challenges in a vacuum, working and talking only to fellow members of this small tribe. These problems require scientific leadership and collective action.
It’s going to take long, hard work. It will require educating several generations about what science is and is not. It will require helping young people see how they can all engage with this way of skeptical, probing, and relentlessly curious thinking. Ultimately, it will require helping young people see science as a tool to help us tackle the big problems we need to solve to create a better world 100 years from now.
Science Policy
A handful of prominent advisors to the White House published a series of articles in the Journal of the American Medical Association on some changes they recommend for how we adapt to the new set of circumstances we’ve been dealt by COVID.
- A National Strategy for the “New Normal” of Life With COVID. JAMA. Jan. 6. (Ezekiel J. Emanuel, Michael Osterholm, Celine R. Gounder)
- A National Strategy for COVID-19. Testing, Surveillance and Mitigation. JAMA. Jan. 6. (David Michaels, Ezekiel J. Emanuel, Rick A. Bright)
- A National Strategy for COVID-19 Medical Countermeasures. Vaccines and Therapeutics. JAMA. Jan. 6. (Luciana L. Borio, Rick A. Bright, Ezekiel J. Emanuel)
- The First 2 Years of COVID-19: Lessons to Improve Preparedness for the Next Pandemic. JAMA. Jan. 6. (Jennifer B. Nuzzo, Lawrence O. Gostin)
- The Pandemic Preparedness Program: Reimagining Public Health. JAMA. Jan. 6. (Eli Y. Adashi, I. Glenn Cohen)
- Covid-19: An urgent call for global “vaccines-plus” action. BMJ. Jan. 3. (An open letter by a group of public health experts, clinicians, scientists)
- Why You Should Upgrade Your Masks to an N95. New York magazine. Interview with Stanford infectious disease doctor Abraar Karan. Dec. 30. (Chas Danner)
Data That Mattered
BridgeBio’s stock crashed after it reported a Phase III trial failed to show a benefit for patients on its drug for transthyretin (TTR) amyloid cardiomyopathy (ATTR-CM). The trial ran for 12 months, and evaluated patients on the 6-minute walk test – how far they can walk in six minutes. Shares fell 70 percent. (Barron’s).
Personnel File
Copenhagen, Denmark and Boston-based Hemab Therapeutics, a company working on bleeding and thrombosis diseases, said John Maraganore will serve as its chairman of the board. Watertown, Mass.-based SQZ Biotechnologies also said Maraganore has agreed to become a strategic advisor. The former CEO of Alnylam Pharmaceuticals also has agreed to be an advisor to Arch Venture Partners and Atlas Venture, a pair of early-stage venture firms that were among the early backers of Alnylam. Maraganore has also agreed to chair a group of advisors to the n-Lorem Foundation, a nonprofit founded by former Ionis Pharmaceuticals CEO Stanley Crooke, which works on antisense oligonucleotide therapies for people with N-of-1 conditions. As if that’s not enough, Maraganore also plans to mentor a number young entrepreneurs, and write the occasional column for Timmerman Report in 2022.
Cambridge, Mass.-based Blueprint Medicines, the developer of cancer drugs, said Kate Haviland is being promoted to president and CEO, from COO, effective Apr. 4. Jeff Albers is moving upstairs to be executive chairman of the board. Haviland will also be joining the board.
Vancouver, BC-based Zymeworks, the antibody drug developer, said Ken Galbraith was brought in as chair and CEO to replace longtime CEO Ali Tehrani. It was an ice cold press release, in which Zymeworks didn’t even thank Tehrani for his service at the company, which he’s led since 2003.
San Francisco-based Spruce Biosciences, the developer of treatments for rare endocrine diseases, hired Javier Szwarcberg as CEO. Samir Gharib, the chief financial officer, was promoted to president and chief financial officer.
Akshay Vaishnaw was promoted to president of Alnylam Pharmaceuticals. He’s been with the company since 2006, in a series of R&D roles of increasing responsibility.
Cathy Friedman, a former biotech investment banker, joined GV as an executive venture partner.

Mira Chaurushiya
Mira Chaurushiya joined Westlake Village Biopartners as a senior partner. She comes from 5AM Ventures. (See Mira in this list of biotech industry leaders who identify as LGBTQ, Timmerman Report, June 2021)
Foster City, Calif.-based Terns Pharmaceuticals hired Jeffrey Jasper as senior vice president, head of research. Terns also promoted Diana Chung to SVP, chief development officer. The company is working on small molecule therapies for non-alcoholic steatohepatitis (NASH).
Deals
Edmonton, Canada-based Entos Pharmaceuticals pocketed a $50 million upfront payment from Eli Lilly. The deal calls for Entos to provide Lilly exclusive access to its proteo-lipid vehicles to deliver nucleic acid therapies to multiple targets in the central and peripheral nervous systems.
Absci announced a research collaboration with Merck. The Vancouver, Wash. company said it will use its Bionic Protein non-standard amino acid technology to produce enzymes for Merck’s biomanufacturing applications.
Illumina agreed to work with SomaLogic, to incorporate a proteomics assay into its next-gen sequencing platform.
Verily agreed to a research collaboration with Sosei Heptares to target GPCRs for immune-mediated diseases.
Novartis formed a three-year research collaboration with Cambridge, Mass.-based Alnylam Pharmaceuticals to develop siRNA drugs against molecular targets implicated in end-stage liver disease.
Amgen struck a partnership with Cambridge, Mass.-based Generate Biomedicines to use machine-learning technology to aid protein drug discovery. Generate will pocket $50 million upfront for initial work on five programs.
Boston-based i20 Therapeutics formed a research collaboration with Janssen Research & Development to work on ionic liquid technology for the oral delivery of macromolecules.
Boston-based Odyssey Therapeutics agreed to acquire Rahko, a quantum machine learning company, to aid with drug discovery. Odyssey raised a $218 million Series A in December. Terms of the acquisition weren’t disclosed.
Financings
San Francisco-based Waymark raised $45 million in a Series A financing co-led by Andreesen Horowitz and NEA. The company is working on helping healthcare providers who accept Medicaid to provide better care to low-income people, by delivering evidence-based medicine at scale to improve outcomes. It’s about going after populations that have been overlooked. According to a company statement:
“Today, the wealthiest Americans outlive the poorest Americans by 10-15 years. The one in four Americans who are on Medicaid are often overlooked by healthcare innovations, reinforcing disparities in health outcomes.”
That second paragraph of the press release shouldn’t be a surprise, but it is interesting to see that healthcare inequities have gotten so deep and pervasive that prominent VC firms don’t look the other way — they now see it as an investable category. See Vineeta Agarwala’s A16Z blog post for more.
Hayward, Calif.-based Eikon Therapeutics said it raised $517 million in a Series B financing. It’s working on super-resolution microscopy and advanced computation to advance drug discovery. T. Rowe Price Associates, Canada Pension Plan Investment Board (CPP Investments), EcoR1 Capital, and UC Investments are among the new investors. CEO Roger Perlmutter is the former president of Merck Research Laboratories. (See my interview with Perlmutter when he joined the company, May 2021)
Cambridge, Mass.-based Korro Bio raised $116 million in a Series B financing to advance its RNA editing platform technology. Eventide Asset Management led. (See this TR feature on Korro and two other RNA editing startups, Shape Therapeutics and Locanabio).
South San Francisco and Paris-based DNA Script, the company developing and marketing benchtop enzymatic DNA synthesis technology, added on a second tranche to its Series C financing, bringing the total from $165 million to $200 million. T. Rowe Price Associates and Baillie Gifford joined the second tranche. (TR coverage of DNA Script, Oct. 2021).
San Francisco-based Esker Therapeutics, a precision immunology company hatched by Foresite Labs, said it closed a $200 million financing led by AyurMaya, an affiliate of Matrix Capital Management, and an unnamed US healthcare fund. The company also changed its name to Alumis. (TR coverage of the company’s plan to develop a best-in-class Tyk2 inhibitor, May 2021).
San Francisco-based Ambagon Therapeutics announced it has raised an $85 million Series A financing led by Nextech Invest. The company is developing molecular glues to go after hard-to-target protein targets.
Carlsbad, Calif.-based Pardes Biosciences debuted as a public company through a SPAC deal sponsored by Foresite Capital, which brought in $274 million in total proceeds. The company is working on oral antivirals for COVID-19.
Seattle-based Curi Bio raised $10 million in a Series A. It’s developing a human stem cell platform for drug discovery.
Seattle-based Good Therapeutics raised $8 million to develop targeted protein drugs, including a targeted IL-2 program.
Our Shared Humanity
- Why the COVID-19 Response Needs International Relations. International Affairs. Sept. 2020. (Sara Davies and Clare Wenham)
- First they ran short of PPE, then ventilators. Now, the shortage is hospital staff. Washington Post. Dec. 30, 2021. (Dan Diamond)
- Two years of COVID-19 in Africa: lessons for the world. Nature. Jan. 3. (Christian Happi and John Nkengasong)
Strategy
- How Early Is Too Early To Establish A Commercial Function In Biotech? LifeSciVC. Jan. 6. (Sush Patel)
Vaccines
Texas Children’s Hospital and Baylor College of Medicine said they received Emergency Use Authorization in India for a protein-and-adjuvant vaccine for COVID-19. The researchers there are partnered with Dynavax for the adjuvant, and with Biological E in India for manufacturing. The vaccine, Corbevax, has shown ability to induce neutralizing antibodies against the original SARS-CoV-2 from Wuhan, as well as the Delta variant, in a pair of Phase III trials that enrolled more than 3,000 subjects. The vaccine hasn’t passed the same kind of large Phase III trials passed by the mRNA and adenoviral vaccines, but it is a cheap and practical technology platform that could help with vaccinations in parts of the world in need. (Washington Post)
Science of Obesity
- Effects on weight loss and glycemic control with SAR441255, a potent unimolecular peptide GLP-1/GIP/GCG receptor triagonist. Cell Metabolism. Dec. 20. (Martin Bossart et al Sanofi)
Science of SARS-CoV-2
- SARS-CoV-2 spike T cell responses induced upon vaccination or infection remain robust against Omicron. MedRxiv. Dec. 28. (Wendy Burgers, Catherine Riou et al, University of Cape Town, South Africa)
- Preserved T cell reactivity to the SARS-CoV-2 Omicron variant indicates continued protection in vaccinated individuals. BioRxiv. Dec. 30. (Lorenzo De Marco et al Santa Lucia Foundation, Italy)
- SARS-CoV-2 vaccination induces immunological memory able to cross-recognize variants from Alpha to Omicron. BioRxiv. Dec. 28. (Alessandro Sette et al, La Jolla Institute for Immunology)
- Ancestral SARS-CoV-2-specific T cells cross-recognize Omicron (B.1.1.529). Research Square. Jan. 3. (Marcus Buggert et al Karolinska Institute)
- Divergent SARS CoV-2 Omicron-specific T- and B-cell responses in COVID-19 vaccine recipients. MedRxiv. Dec. 29. (Rory de Vries et al Erasmus MC, The Netherlands)
- Clinical Severity of COVID-19 Patients Admitted to Hospitals in Gauteng, South Africa During the Omicron-Dominant Fourth Wave. The Lancet preprints. Dec. 29. (Lucille Blumberg, Cheryl Cohen et al National Health Laboratory Services (NHLS) – National Institute for Communicable Diseases, South Africa)
This Week in Drug Pricing
Reports from GoodRx and analysts at Raymond James estimate that pharma list prices are rising by about 5 percent at the start of 2022. That’s not outrageous on its face, especially because it doesn’t take into account rebates and discounts that are almost always hidden from view. But these numbers are all being calculated off of high baselines that really aren’t sustainable. See an overview of the macro pricing data from BioPharma Dive.
Last week, I wrote here optimistically that Novartis won FDA clearance for inclisiran (Leqvio), the siRNA molecule aimed at PCSK9 for lowering cholesterol. With fewer injections and a low price, it could reach many millions of patients and reduce their risk of heart attack, stroke and death from cardiovascular disease. So I was disappointed to read the follow-up this week by John LaMattina in Forbes. Novartis set the price at $3,250 per dose — $9,750 in year one and $6,500 each year after. That’s More, not Less, than the competing antibody drugs from Sanofi/Regeneron and Amgen. It’s a missed opportunity to change business as usual.
In case anyone needs a reminder, there are a lot of people out there suffering from financial hardship in the US after they get sick. See this Jan. 4 article in the Journal of the National Cancer Institute. It looked at 380 metastatic colorectal cancer patients – 77.7 percent of them white, and 98 percent of them insured. Researchers led by Veena Shankaran at the Hutchinson Institute for Cancer Outcomes Research found that nearly 3 out of 4 of these patients suffered a Major Financial Hardship – “defined as 1 or more of increased debt, new loans from family and/or friends, selling or refinancing home, or 20% or more income decline.” The researchers conclude that the results “underscore the need for clinic and policy solutions that protect cancer patients from financial harm.” I’d like to expand that protection beyond cancer patients, when we get serious about what’s broken in US healthcare.
Biogen is still trying to sell an unproven Alzheimer’s medicine at the still-hefty price tag of $28,000 a year. The next big act in the Aduhelm saga is an important reimbursement decision by the Center for Medicare and Medicaid Services (CMS). Peter Bach weighed in with an editorial in Bloomberg, calling for CMS to demand further testing before it pays the bills.
A large majority of the American public, consistently about 70 percent or more, says it is fed up with high drug prices. Many are demanding the Build Back Better legislation being negotiated on Capitol Hill put drug prices on some kind of leash. It’s not an unfounded concern. Like a lot of people, I want to see some sensible policy that can provide a safety net for people who get sick in this country, so they aren’t terrified of going broke. One big reason I was able to start Timmerman Report seven years ago is that I’m healthy, my family is healthy, and I don’t need to go take a job at Microsoft just to get health insurance. Our system right now is perverse and stupid and cruel. It puts way too much burden on individuals. But I also don’t want to see some ham-fisted over-correction that takes away the incentive to spend a lot of time and money on risky biomedical R&D. That would hurt a lot of people in the long term as well. Peter Kolchinsky and Michael Gilman wrote this week about some of the problems with the current legislation, especially the part that would disincentivize the development of small molecules. See their Jan. 4 editorial in The Well News. For a chaser, see also Alex Harding’s Jan. 3 TR article on why this is actually an auspicious time to invest in small molecule development.
The Best of Times
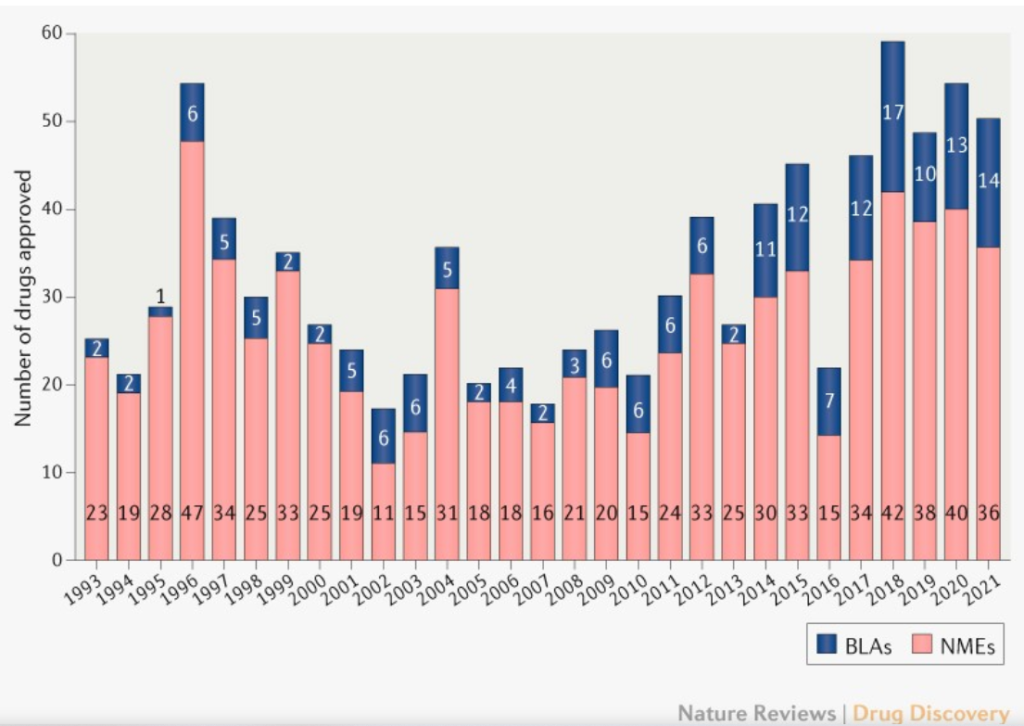
The end of a year always brings articles on the number of New Chemical Entities, the number of novel new drugs, approved by the FDA. This year, there were 50 such approvals, according to a tally by Asher Mullard at Nature Reviews Drug Discovery. The trend is clear over the first two decades of the 21st century. There are a lot more scientifically interesting and medically meaningful therapies coming down the pike. If we can figure out how to more fairly distribute the progress here and around the world, and thread the needle on fair prices that incentivize creators, we can probably expect this trend to continue heading up and to the right.
The vaccines took our breath away with their outstanding balance of safety and efficacy, and the seemingly speed at which they were developed. That’s story has been, and is being, well told. But the development of Paxlovid, an oral protease inhibitor that works against all known variants, is another tour de force that could save millions of lives. John LaMattina, a former president of Pfizer R&D, interviewed one of the unsung heroes of that program at today’s Pfizer, Dafydd Owen.
An Opportunity for Grownups
Some people raised objections back in October about whether kids ages 5-11 should be getting the Pfizer / BioNtech COVID-19 vaccine. They said they weren’t sure it was safe, given the reports of myocarditis in adolescents, especially adolescent and young men. Pfizer / BioNtech cut the dose down from 30 micrograms to 10 micrograms for the kids. Now we have more data. At last count, about 8.7 million shots were given to kids ages 5-11.
That’s a big real-world database to cull through to look for potentially rare side effects of consequence.
What did the CDC find? See the following key line in the Dec. 31 edition of the Morbidity and Mortality Weekly Report:
“Among 15 preliminary reports of myocarditis identified during the analytic period, 11 were verified (by provider interview or medical record review) and met the case definition for myocarditis; of these 11 children, seven recovered, and four were recovering at time of the report.”
In other words, myocarditis is less than a one-in-a-million event in kids with the low dose. That’s an extremely rare risk. Yet here we are, and the vast majority of kids in this age group are still unvaccinated. As I wrote in the Dec. 9 Frontpoints, this lack of vaccination is a missed opportunity for grownups. There is still time for kids to get the shot, which will keep them from getting severely sick with COVID and reduce the probability that they’ll transmit the infection to more vulnerable adults.
Legal Corner
Elizabeth Holmes was found guilty on four charges of defrauding investors during her time at the helm of Theranos, the blood testing company. I’m glad to see that crime didn’t pay for her, as it often does for many people in the public eye. Now that the jury weighed the evidence, I hope that we can banish Ms. Holmes from the attention economy. She sucked up way too much oxygen, attracted too many clicks, and distracted people from considering real issues and solving real problems. I think there was little to see here other than a bunch of people who got bamboozled. The takeaway is common sense — investors ought to do their own diligence before writing checks.

Debbie Nickerson
RIP
Debbie Nickerson, a professor of genome sciences at the University of Washington, and a leading researcher in human genetics over the past 30 years, died just before Christmas. She had an aggressive abdominal cancer.
Tweetworthy
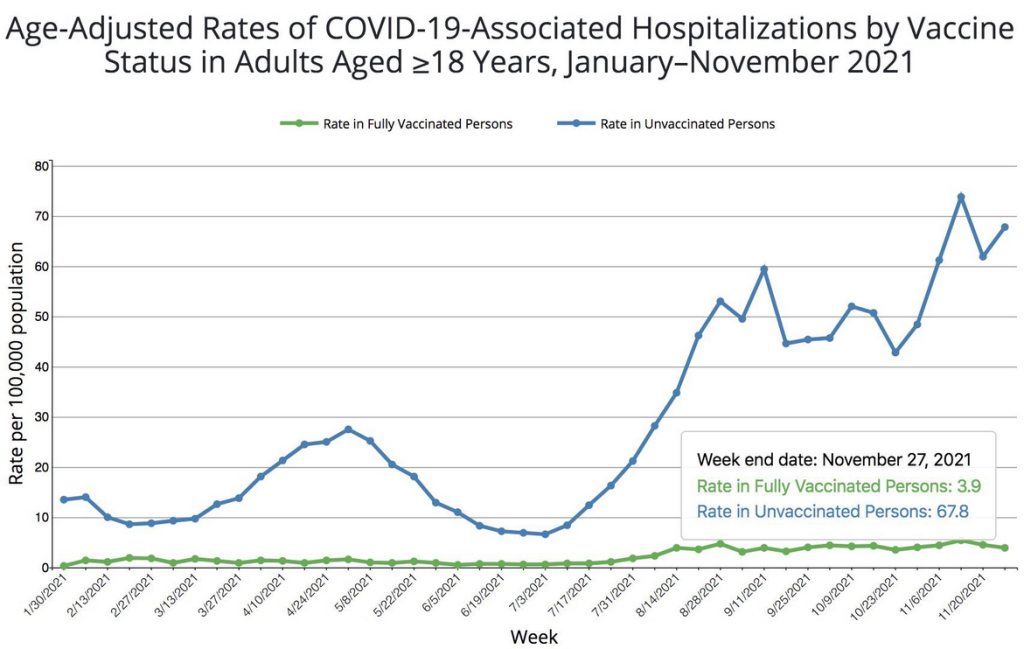
Are the vaccines doing any good? See data below from the White House Chief of Staff, and shared on Twitter by former FDA commissioner Scott Gottlieb.