A Historic Nobel for CRISPR, Pfizer Sticks Up for FDA, & a CDC Legend Speaks

Luke Timmerman, founder & editor, Timmerman Report
What a time to be alive in biomedicine. Catch up on the main events in Frontpoints.
The Babe Ruth of Public Health Speaks Out
William Foege is in his mid-80s. There’s a genome sciences building at the University of Washington named after him because Bill Gates gave money to build it, and he wanted it named after a scientific hero. Foege has nothing to prove to anyone, and doesn’t seek attention. He’s belongs on the Mount Rushmore of global health for his strategically brilliant and relentless campaign to eradicate smallpox from Earth.
So I snapped straight up and read every word Foege wrote when USA Today discovered and published a private letter Foege wrote to current CDC director Robert Redfield, dated Sept. 23.
Foege empathizes with the embattled CDC director in the opening, but like an angry and baffled father, he chastised Redfield for this monumental failing of American leadership in public health. The only real alternative at this point, Foege wrote, is for Redfield to document the horrifying cascade of White House incompetence and mendacity, and resign to save the CDC, a once-proud and now humiliated agency.
Read the full, scathing letter here.
CDC, Finally, Updates Airborne Guidance
We’ve known since January that SARS-CoV-2 is transmitted through the air. There have been debates over the relative extent of droplets versus aerosols, and how far is far enough for social distancing, but still, but the science on tiny aerosol transmission has been clear for some time. And yet, the CDC, has been unable to effectively communicate this to the public. Remember when Dr. Redfield told a Congressional committee that a mask was an even more effective defense tool than a vaccine, and when the President slapped him down for that act of fact-based insubordination? The latest shameful episode in CDC history came when the agency briefly took down guidance from its website in September that emphasized the risk from airborne transmission of the virus. It took until Oct. 5 – yet another day with more than 40,000 new cases – before the CDC was able to update its guidance on its website about airborne transmission. Look at the terrible case of confusing whiplash this gives the public, neatly captured in WSJ editor Jonathan Rockoff’s tweets.

If citizens had been given the straight scoop, straight from the podium of the CDC starting in January and continuously updated from that point forward, I firmly believe this country would have looked more like Germany today. We would have been able to save tens of thousands of lives. But now, as things are, many thousands more are going to die – still 1,000 a day! This horse should never have gotten so far out of the barn. The body politic should never have been so thoroughly poisoned that we’d up in a cynical abyss where large numbers of citizens refuse to wear masks and engage in common sense social distancing.
All of the elected officials who are responsible, and those who continue to enable this daily rolling national train wreck, must be voted out of office.
Kudos
The Nobel Prizes came out, and were quite illustrative of the golden age of biomedicine. On Monday, the Nobel Prize in Physiology or Medicine went to Harvey Alter at the National Institutes of Health; Michael Houghton, now at the University of Alberta; and Charles Rice, now at Rockefeller University. Their work on the hepatitis C virus laid the groundwork for one of the biggest success stories in biotech history – cures for this chronic, liver-damaging infection. Houghton, by the way, did his pioneering work on HCV while at Chiron, one of the early biotech companies (now part of Novartis).
Then, on Wednesday, came an even bigger award – the Nobel Prize for Chemistry went to Jennifer Doudna and Emmanuelle Charpentier for their discovery of CRISPR-Cas9 gene editing. This discovery of precise editing tools was revolutionary, and is now setting the stage for a new crop of gene-edited medicines with potential to cure diseases like sickle-cell disease, a new generation of drug discovery platforms to speed up precision medicine development, clever agricultural applications, cheap and fast diagnostics and so much more. The cheap, fast, easy nature of CRISPR editing makes it an accessible lab tool widely distributed in labs around the world. Doudna and Charpentier’s contribution not only changed biomedicine, but this dynamic duo is especially inspiring to women scientists and young girls. That was the bigger story than the long-running patent dispute between the University of California-Berkeley and the Broad Institute. George Church and Eric Lander, a couple of leading lions of science with competing claims to novelty in the early work on CRISPR, both made gracious public comments, rather than resort to the kind of occasionally ugly professional jealousy that often swirls around the Nobels. Church, when asked about Doudna and Charpentier’s prize, told STAT “they made the key discovery.”
Isaac Kohane, a professor of biomedical informatics at Harvard Medical School, was awarded the 2020 Morris F. Collen Award of Excellence from the American College of Medical Informatics.
Regulatory Action
The FDA released guidance for COVID-19 vaccine development, and what the standards are for an Emergency Use Authorization, in advance of the upcoming Oct. 22 advisory committee to review the latest safety and efficacy data on this historic effort. The big news in the document (full version here) was that the FDA is calling for at least two months of safety data (see Appendix II) from participants in trials after getting their second and final dose. That means there’s no way an EUA can be issued before the general election on Nov. 3. The White House had reportedly balked at the guidance document, and held it up with questions for FDA. The White House has done considerable damage to the FDA’s reputation for scientific independence this year, but as the President was ill with COVID-19, the White House ultimately backed off, dropping its objections to the balance the agency has been trying to strike between the need for speed, as well as the need for adequate safety data. Given the state of vaccine hesitancy in the public, which perceives a corner-cutting job in the works for electoral purposes, we can be thankful that cooler heads have prevailed.
Albert Bourla, the CEO of Pfizer, may have had a hand in the White House backing off from its aggressive stance, as he said unequivocally that his company wouldn’t try to undercut the independence of the FDA (even if it’s presumably financially advantageous for the company to get a jump on rivals and start selling its COVID vaccine as fast as possible). See his Tweet below.
One final point about COVID-19 vaccine timelines that has gotten less attention: waiting just a few weeks longer for the trials to play out could be the difference between making a momentous decision on a rather skimpy data set, or making it based on a fully mature dataset rich with information on key subgroups like the elderly, and racial and ethnic groups that have been reluctant to enroll in trials (and who especially need vaccines because of the higher-than-average risk minority groups face.) See this comment I tweeted in the moment from Larry Corey of Fred Hutch, one of the leaders of the Operation Warp Speed effort, who spoke at a Johns Hopkins University and University of Washington symposium on Oct. 6.

Tarrytown, NY-based Regeneron Pharmaceuticals submitted a request Oct. 7 to the FDA for Emergency Use Authorization for its REGN-COV2 two-antibody cocktail that’s designed to neutralize the SARS-CoV-2 virus. This comes after the company issued a press release Sept. 29 with the first meaningful slice of clinical data, showing the antibody could significantly reduce viral loads, especially among patients with high amounts of circulating virus. Data from that rolling clinical program haven’t yet been published in a peer-reviewed journal. The move to seek an EUA for wide distribution to patients came the same day the President extolled the virtues of the therapeutic neutralizing antibody from Regeneron, which he received on a Compassionate Use basis, and which he publicly credited with helping him recover from COVID-19. Whether that’s true, of course, we don’t know.
New York-based Mesoblast received a dreaded Complete Response Letter from the FDA, explaining why it wasn’t willing to approve the company’s application to market a new treatment for pediatric steroid-refractory graft-versus-host-disease in children. This was a surprise, given that the FDA’s Oncologic Drugs Advisory Committee voted 9-1 in favor, saying the available data support the drug’s efficacy. The FDA disagreed, saying the company should run at least one additional randomized study in adults or children.
New York-based Y-mAbs Therapeutics received a Refusal to File letter – meaning the FDA declined to review its application to start marketing omburtamab as a treatment of pediatric patients with CNS/leptomeningeal metastasis from neuroblastoma. The agency said the company needs to provide more detail in its Chemistry Manufacturing and Control (CMC) section, and the Clinical section of the Biologics License Application.
San Carlos, Calif.-based Iovance Biotherapeutics reported that its plans to file a Biologics License Application to the FDA have been delayed, based on a disagreement with the agency over “the required potency assays to fully define its TIL therapy.” The company is developing lifileucel as a Tumor-Infiltrating Lymphocyte therapy for metastatic melanoma.
Deals
Bristol-Myers Squibb agreed to pay $13.1 billion in cash, or $225 a share, to acquire Brisbane, Calif.-based Myokardia, the developer of targeted therapies for cardiovascular diseases. Through the deal, BMS obtains mavacamten, a first-in-class treatment for obstructive hypertrophic cardiomyopathy which has gone through Phase III development, and which is expected to go before the FDA with a New Drug Application in the first quarter of 2021. Third Rock Ventures bet $38 million on this company in a Series A financing in September 2012 (see my coverage at the time on Xconomy.)
Pleasanton, Calif.-based 10X Genomics acquired Boston-based ReadCoor for $350 million, a few weeks after its acquisition of Stockholm-based CartaNA. The two acquisitions represent a strategic move into In Situ analysis. (See TR coverage of ReadCoor in this June 2020 feature on “Complexity in Motion.”)
Vancouver, BC-and Seattle-based Chinook Therapeutics closed its merger with Aduro Biotech and completed a $115 million private placement that leaves it with $275 million in cash to develop precision medicine therapies for kidney diseases.
Palo Alto, Calif.-based BridgeBio Pharma agreed to pay $175 million to acquire the remainder of Eidos Therapeutics which it didn’t already own. Eidos is developing acoramidis, a TTR stabilizer for patients with ATTR cardiomyopathy and polyneuropathy.
Cambridge, Mass.-based AVROBio in-licensed a lentiviral gene therapy program from the University of Manchester, to treat patients with Hunter Syndrome. (See TR coverage of AVROBio, September 2020)
The Netherlands and Belgium-based argenx announced a series of partnerships with Chugai Pharmaceuticals, The Clayton Foundation, and Halozyme Therapeutics to expand its antibody engineering capabilities.
Takeda Pharmaceuticals and Arrowhead Pharmaceuticals agreed to work together on further developing ARO-AAT for Alpha-1 Antitrypsin-Associated Liver Disease. Arrowhead is getting $300 million upfront.
Data That Mattered
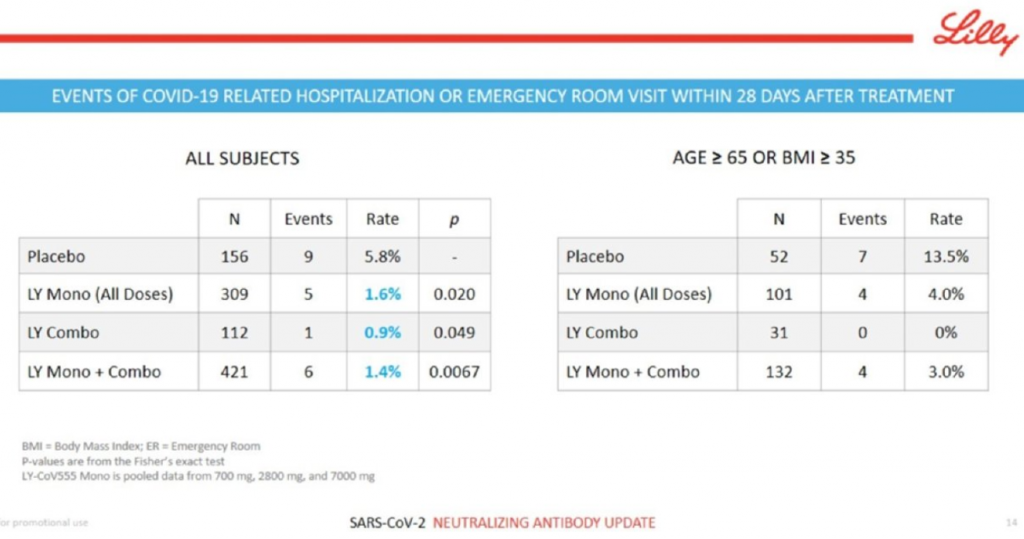
Eli Lilly had the best clinical data of the week, hands down. The Indianapolis-based pharma company reported that its neutralizing antibody program for COVID-19, developed in collaboration with AbCellera against the spike protein of SARS-CoV-2, nailed an interim analysis of the BLAZE-1 clinical trial that looked at mild-to-moderate COVID-19 patients. The combo therapy of two antibodies did especially well at taking down viral loads at Day 3, Day 7 and Day 11, and reduced COVID-related hospitalization and ER visits. The company said it will seek Emergency Use Authorization from the FDA based on the result. This is the kind of biological and clinical data alignment that we all want to see. Key slide below.
Amgen had a mixed bag of recent results. The company reported positive top-line results for its KRAS G12C directed small molecule drug candidate, sotorasib, in a Phase II study of 126 patients with KRAS G12C mutated forms of advanced non-small cell lung cancer. A few days later, Amgen reported on a failed Phase III result for omecamtiv mecarbil in patients with heart failure with reduced ejection fraction (HFrEF). Technically, the trial hit the primary composite endpoint, but the benefit on that score was miniscule, and the drug failed to demonstrate an improvement in cardiovascular death, a key secondary endpoint. Results from the GALACTIC-HF study, which enrolled, 8,256 patients in 35 countries, will be presented at the American Heart Association’s Scientific Sessions on Nov. 13.
Gilead Sciences and collaborators from the National Institute of Allergy and Infectious Disease published final results for remdesivir in the New England Journal of Medicine. The full analysis confirms the earlier reports that the antiviral can help patients recover more quickly, and get out of the hospital faster. The drug didn’t show a statistically significant benefit on mortality at Day 29, but a post-hoc analysis showed there was a 72 percent mortality benefit in a subgroup of patients who got low-flow oxygen upon hospital admission.
Science
- Airborne Transmission of SARS-CoV-2. Science. Oct. 5. (Kimberly Prather et al)
- Cross Reactive Memory T-cells and Herd Immunity to SARS-CoV-2. Nature Reviews Immunology. Oct. 6. (Marc Lipsitch et al)
- Antigen-based Testing, But Not Real-Time PCR, Correlates With SARS-CoV-2 Virus Culture. MedRxiv. Oct. 5. (Andrew Pekosz et al)
- Remdesivir for the Treatment of COVID-19. Final Report. New England Journal of Medicine. Oct. 8. (John Biegel et al)
- Effect of Hydroxychloroquine in Hospitalized Patients With COVID-19. New England Journal of Medicine. Oct. 8. (The RECOVERY Collaborative Group)
- Remdesivir Targets a Structurally Analogous Region of the Ebola and SARS-CoV-2 Polymerases. Proceedings of the National Academy of Sciences. Oct. 7. (Michael Lo et al)
Science Features
- Charting a COVID19 Immune Response. NYT. Oct. 5. (Katherine Wu and Jonathan Corum)
- LongCovid: Let Patients Help Define Long-Lasting Symptoms. Nature Editorial. Oct. 7. (The Editors)
- How Superspreading Events Drive Most COVID-19 Spread. Scientific American. June 23. (Christie Aschwanden)
- How the White House Flouted Basic Coronavirus Rules. NYT. Oct. 8. (Lauren Leatherby et al)
Public Health
- Get Serious About the People Putting All of Us at Risk. Let’s Enforce Mask Wearing. NYT. Sept. 29. (Elisabeth Rosenthal)
- Against COVID-19, Imperfect Measures Do the Most Good. NYT. Oct. 4. (Joshua Schiffer)
Strategy
- Playing to Win: Scenario Planning for a Binary Readout in Biotech. LifeSciVC. Oct. 8. (Ankit Mahadevia)
Politics
- Why Nature Needs to Cover Politics More Than Ever Now. Nature Editorial. Oct. 6. (The Editors)
- Dying in a Leadership Vacuum. New England Journal of Medicine Editorial. Oct. 8. (The Editors)
- Coronavirus Advisor Scott Atlas Hits Back at Fauci and Others Who Doubt His Advice. “I’m Here Because the Country’s Off the Rails.” Business Insider. Oct. 6. (Ashley Collman)
Worth a Listen
- Moncef Slaoui, The Man Behind America’s Race for a Coronavirus Vaccine. Sway podcast. Oct. 5. (Kara Swisher)
Personnel File
Merck said that Roger Perlmutter, president of Merck Research Laboratories since 2013, is retiring on Jan. 1, 2021. He will be replaced by Dean Li, the company’s senior vice president of discovery sciences and translational medicine. Li is a former professor of medicine and cardiology at the University of Utah. Perlmutter, an immunologist by training, presided over the breakout success of the cancer immunotherapy pembrolizumab (Keytruda).
Brett Zbar joined General Atlantic as global head of life sciences, as the growth equity firm formally established life sciences as its fifth core investment sector. Zbar was previously a managing director with Foresite Capital. (I wrote about Zbar in 2018 as one of “Nine VCs Who Matter, But You Never Read About.”)
Paul Medeiros joined Cambridge, Mass.-based Q-State Biosciences as president and CEO. The company is developing drugs for diseases associated with electrically excitable cells.
San Rafael, Calif.-based BioMarin Pharmaceutical hired Kevin Eggan as Group Vice President, Head of Research and Early Development. He was formerly a tenured professor in the Department of Stem Cell and Regenerative Biology at Harvard University, the Director of Stem Cell Biology for the Stanley Center for Psychiatric Research at the Broad Institute, and an Institute Member of the Broad Institute of MIT and Harvard.
San Diego-based Expansion Therapeutics named Renato Skerlj as its new CEO. The company is developing small molecule drugs against RNA targets.
Sarah Noonberg was hired as chief medical officer of San Francisco-based Maze Therapeutics. (See TR coverage of the $191 million Series A in this company in February 2019, led by Third Rock Ventures and Arch Venture Partners.)
Rick Bright, the former head of the Biomedical Advanced Research and Development Authority who became a whistleblower on the political interference in the COVID-19 response effort, including the unwarranted push for hydroxychloroquine, resigned. In his amended whistleblower complaint, he said he had only been given one assignment since being transferred to the NIH.
Norwood, Mass.-based Corbus Pharmaceuticals cut its workforce by 54 percent.
Name Change
Seattle Genetics officially changed its name to SeaGen Inc. – which has been its informal name for years. The company is actually based in suburban Bothell, Wash. and isn’t exactly a genetics company, it’s a cancer drug developer. It’s never made a lot of buzz for itself, but it has long been the unquestioned bellwether of the Seattle biotech hub with a $35 billion market valuation.
Financings
San Mateo, Calif. and Cambridge, Mass.-based Kronos Bio, a cancer drug developer led by former Gilead CSO Norbert Bischofberger, raised $250 million in an IPO at $19 a share.
Watertown, Mass.-based C4 Therapeutics, the developer of targeted protein degrading drugs, raised $210 million in an IPO at $19 a share.
Silver Spring, Maryland-based Aziyo Biologics raised $50 million in an IPO at $17 a share.
Durham, NC and Austin, Tex.-based Shattuck Labs, a cancer drug developer, raised $202 million in an IPO at $17 a share.
Exton, Penn.-based Immunome, a company working on memory B-cells to develop antibody therapies, raised $39 million in an IPO at $12 a share.
Cambridge, Mass.-based Oncorus, a viral immunotherapy developer for cancer, raised $87 million in an IPO at $15 a share.
Alameda, Calif.-based Scribe Therapeutics raised $20 million in a Series A financing to develop in vivo CRISPR gene editing medicines. Andreesen Horowitz led. It spun out of the Doudna Lab, Oakes Lab, and the Innovative Genomics Institute. Scribe also announced a Biogen partnership to work on neurodegeneration.
South San Francisco-based Federation Bio got started with a $50 million Series A to develop microbiome therapies. Horizons Ventures led, and was joined by existing investors Venrock and Altitude. Emily Drabant Conley, formerly of 23andMe, was named CEO.
Stamford, Conn.-based Springworks Therapeutics, a cancer drug developer, raised $250 million in a stock offering at $51 a share.





