How Worried Should We Be About Emerging Strains of SARS-CoV-2?

Mara Aspinall, managing director, BlueStone Venture Partners; professor of the practice, biomedical diagnostics, Arizona State University
All virus strains mutate continuously. That’s normal. There’s nothing inherently concerning about that word, “mutation.”
Unless, of course, the mutations give an evolving virus new properties that make it more transmissible or more pathogenic. That’s trouble.
The news has been worrisome in recent weeks with reports on the B.1.1.7 strain first detected in the UK, and other new strains of SARS-CoV-2, such as the E484K strain first identified in South Africa.
Before getting into the particulars of those new strains, let’s go over some background. Large numbers of silent mutations are accumulated over time with all viruses. These silent mutations, which don’t cause meaningful change in the clinical sense, are helpful to us because they create a viral fingerprint. This unique code can be used to track the spread of particular versions over time and by geography — the data that tells us how and where a particular virus emerged.
When a mutation persists and grows as a cause of infection there are two possible reasons: pure chance — that mutation just happened to be in the right place at the right time (aka founder effect); or, more worryingly, because it is more virulent or fitter than its ancestors. The vast majority of mutations do not persist, but a very few do, and when that happens, we have to quickly figure out how concerned to be, and what to do to outmaneuver the fast-spreading virus.
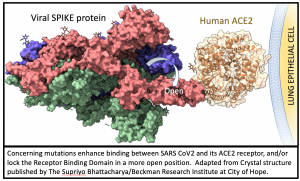 All mutations that are incorporated into virus strains that spread out geographically and grow faster than their predecessors are a “smoking gun”. This is exactly what has happened in the United Kingdom over the past three months and may be happening again in South Africa. The most recent analysis of the UK strain (B.1.1.7; aka VOC-202012/01; aka 20B/501Y.V1) combines prior laboratory findings (Scott 2020) and animal data (Gu 2020) that the N501Y spike enhances the critical spike protein’s ability to bind its human receptor (ACE2).
All mutations that are incorporated into virus strains that spread out geographically and grow faster than their predecessors are a “smoking gun”. This is exactly what has happened in the United Kingdom over the past three months and may be happening again in South Africa. The most recent analysis of the UK strain (B.1.1.7; aka VOC-202012/01; aka 20B/501Y.V1) combines prior laboratory findings (Scott 2020) and animal data (Gu 2020) that the N501Y spike enhances the critical spike protein’s ability to bind its human receptor (ACE2).
This finding has serious real-world implications. We see it in the recent explosive growth of UK cases of this strain (rapidly approaching complete dominance). The conclusion is that the UK strain is 50% to 75% more infective than the earlier strains in circulation, boosting its reproduction number Rt from ~1 towards 2.
At the very least, that means that the clock has sped up, and our time window to react effectively is closing. We must dramatically speed up our response – more and more frequent testing; better mask/distance/ventilation/handwashing compliance; faster vaccine roll-out than achieved to date.
The UK and South Africa strains are not the first major variant strains we have faced, but they are the most serious – direct evidence we are living on borrowed time. There have been four major strains of concern since early 2020: the D614G mutation in February; the UK strain in September; the Danish Mink crisis in November; and in December – the South Africa strain (E484K). Each strain is comprised of a number of variants, what follows is a brief summary of the key mutations believed to underly our greatest threat:
February – variant D614G
Extensive in-vitro and mouse work (Plante 2020) confirmed that this strain was more highly transmissible than the original Wuhan strain, most likely because this specific mutation created a more “open” and therefore more infective spike protein. This mutation is now globally dominant as a result and is the common ancestor of more recent strain emergence, against which they are evaluated.
September – UK strain N501Y spike variant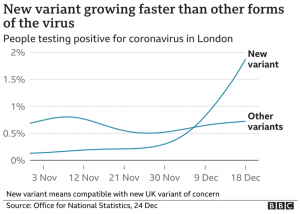
The UK has a national and comprehensive sequencing initiative (the US has a decentralized approach). Ironically, this greater effort at genomic surveillance in the UK may amplify concern in the absence of good data elsewhere. Since its identification in the UK, many other countries, including the US, have found it locally.
This strain comprises 17+ protein-altering variants, six of which are in the Receptor Binding Domain (RBD) of the spike protein, one of which (N501Y) is known to increase virus binding affinity and infectivity. Highly specific monoclonal antibodies may show less therapeutic binding and may be less effective.
The vast majority of new cases in London and the south east UK are of this strain. Higher rates of infection in children are being reported. Population transmission fitness is about 47-75% greater. Spike-based PCR tests are reporting spike negative results, and this is being used as a biomarker of strain prevalence. It is unlikely this strain is dominating only by chance.
November – Danish Mink Y453F variant (aka “Cluster 5”)
It is a little unfair to label this the Danish strain since it was also found in Dutch, Spanish, Swedish and US mink. Among the four spike mutations comprising this strain, Y453F was found (Scott 2020) to be second only to N501Y in ability to enhance spike binding. There is no evidence that this strain is either more severe or transmissible. No increase in human cases accompanied the emergence of this strain, so the current hypothesis is that its emergence is more likely a chance-driven event. However, it is most concerning since an animal-to-human pathway (i.e. via mink) is a potential avenue for further threatening mutations to arise.
December – South Africa strain E484K and K417N plus N501Y (aka 501.V2; aka B.1.351)
The least is known about this strain, which has only recently been identified. It consists of 21 mutations, nine of which are in the spike protein. It appears to share the UK strain’s greater transmissibility, but this has not been reliably quantified, and there is no evidence today of enhanced severity. However, this is the strain which presents the most concern with respect to the current vaccines, given in-vitro studies have suggested some decreased efficacy of neutralizing antibodies in the presence of the E484K mutation (Weisblum 2020; Adreano 2020)
Although we have very limited reliable data on which to evaluate the impact of each of these individual strains, action cannot wait.
Specifically, there are five areas of concern.
Will these emerging variants:
- Make tests less accurate, threatening our ability to contain the pandemic?
- Make the COVID-19 pandemic worse by increasing cases and deaths?
- Make emerging treatments ineffective?
- Make vaccines ineffective?
- Make the vaccination threshold for achieving herd immunity higher?
The best current answers are:
- Make tests less accurate? No for antigen tests because all antigen tests cleared via the FDA’s Emergency Use Authorization target the abundant N (nucleocapsid) protein, not the spike protein. PCR and serology tests are more vulnerable since they rely on short stretches of RNA for primers and probes, or limited antigen protein segments, respectively, either one of which may by rendered less effective in the face of novel mutations. However, only a very few target spike RNA at all, and those that do sample additional regions and genes in combination (e.g. Thermo-Fisher Taqpath PCR assay). Few manufacturers publish which antigens are utilized in serology tests – so more investigation is needed to determine the efficacy of serology assays. Overall, PCR and antigen based active-disease testing is likely minimally affected.
- Increase cases and deaths? Almost certainly. None of the known variants have been shown to increase case fatality rates (CFR, percent of infected who die). However, if the greater transmissibility of UK variant associated mutations is confirmed, then the resulting rise in new cases will threaten to overwhelm hospital capacity at an even faster rate, which will lead to more total deaths.
- Render treatments Ineffective? Convalescent serum — less likely; monoclonal antibodies — some yes. Convalescent serum has a broad spectrum of antibodies, and although its utility may be limited, it is less likely to be affected than cloned single epitope monoclonals if directed at epitopes directly modified by the new variants. It’s worth noting here that the Regeneron antibody cocktail was specifically designed to hit two epitopes, making it more difficult for the virus to evolve an escape mechanism.
- Will vaccines remain effective? Yes, current vaccines are likely still effective. Both currently authorized vaccines in the US invoke immunity against the entire spike protein, i.e. polyclonal responses, so a few changes among all 1,273 amino acids in the protein are unlikely to reduce vaccine effectiveness. Recent unpublished work (Xie 2021) confirms that the Pfizer vaccine (BNT162b2) raises comparable levels of antibodies to the N501Y mutated (Y) virus as to the prior (N) virus. Testing of the Moderna vaccine is underway. All our current data comes from laboratory work on single mutations. It is possible that a novel group of mutations (e.g. the South Africa strain) may have the potential to enable immune escape by the strain. The good news is that even if current vaccines do need to be modified, a new generation of mRNA vaccines can be quickly created (months not years) to adapt.
- Will increased vaccination be needed? Likely yes. Achieving herd immunity – the ultimate objective of pandemic control – is a function of both viral contagiousness and vaccine efficacy.[1] If more transmissible strains become widespread, the proportion of global population vaccination required for herd immunity increases, and with it the number of vaccines that must be distributed and administered. The SARS CoV2 vaccine roll-out has to be even quicker and broader than has been previously assumed, and we are falling behind even those more modest expectations. Time is not on our side.
Mara Aspinall is co-founder and managing director of BlueStone Venture Partners and a professor of the practice, biomedical diagnostics, at Arizona State University.
[1] Herd immunity for highly contagious measles requires 95% of children to receive the 97% effective vaccine; but for the less contagious influenza, herd immunity is achieved with only 50% being vaccinated with at most a 60% effective vaccine (e.g. the 2010/11 season) or 80% for more typical 30-40% effective annual vaccines.



