Get In-depth Biotech Coverage with Timmerman Report.
21
Nov
2024
Dr. Oz to CMS, Amgen and Pfizer Get New CSOs, & SubQ Keytruda Arrives
Please subscribe and tell your friends why it’s worthwhile. Quality journalism costs money. When you subscribe to Timmerman Report at $199 per year, you reward quality independent biotech reporting, and encourage more.











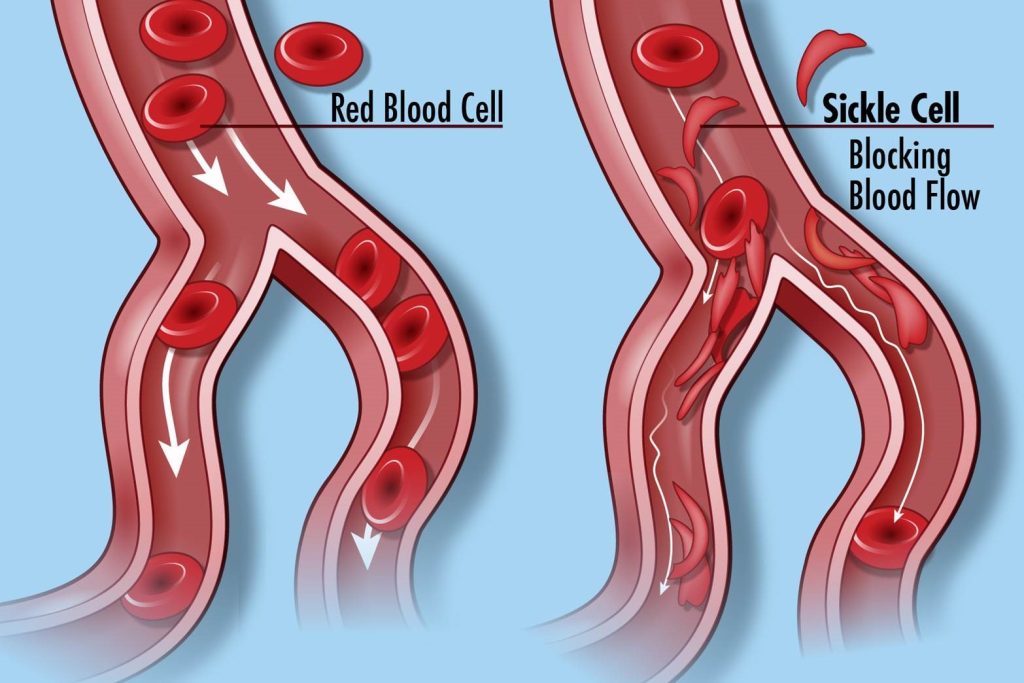








 I have always kept my business and political lives separate – and have had no trouble doing so. But now my political beliefs and my industry are threatened by the same plague.
I have always kept my business and political lives separate – and have had no trouble doing so. But now my political beliefs and my industry are threatened by the same plague.