The Insurrectionist and the Visionary

Luke Timmerman, founder & editor, Timmerman Report
One photograph captured our contradictions on Jan. 6.
There was the man carrying the Confederate flag where it had never flown before – inside the US Capitol.
A violent mob, carrying symbols like that and worse, sought to assassinate elected officials and overthrow our democracy. They were sent there by other elected leaders who were telling lies.
It was horrific.
But look closer at the photo. There’s a portrait on the wall of a true American visionary — Justin Smith Morrill.

Kevin Seefried holds a Confederate flag outside the Senate Chamber during a riot in the U.S. Capitol on Jan. 6. (Associated Press)
Who?
US Rep. Morrill, from Vermont, was a founding member of the Republican Party. His signature achievement was the Land Grant College Act of 1862, also known as the Morrill Act. The law allowed proceeds from federal land sales to benefit states. The states, the ones still in the Union at the time, could use the bounty to start up technical and agricultural colleges. The law contained a community outreach component, and Reserve Officer Training Corps (ROTC) programs for decentralized military training across the states.
The bill had stalled for years in Congress, but was finally passed at the height of the Civil War. It was signed into law by President Abraham Lincoln.
This turned into one of the best investments in American history. MIT, Cornell University, the University of Wisconsin-Madison, University of California-Berkeley, and Michigan State University are a few of the outstanding education and research centers that owe their existence to this act (see map). Years after the war, former Confederate states were able to participate, as were the Historically Black Colleges and Universities.
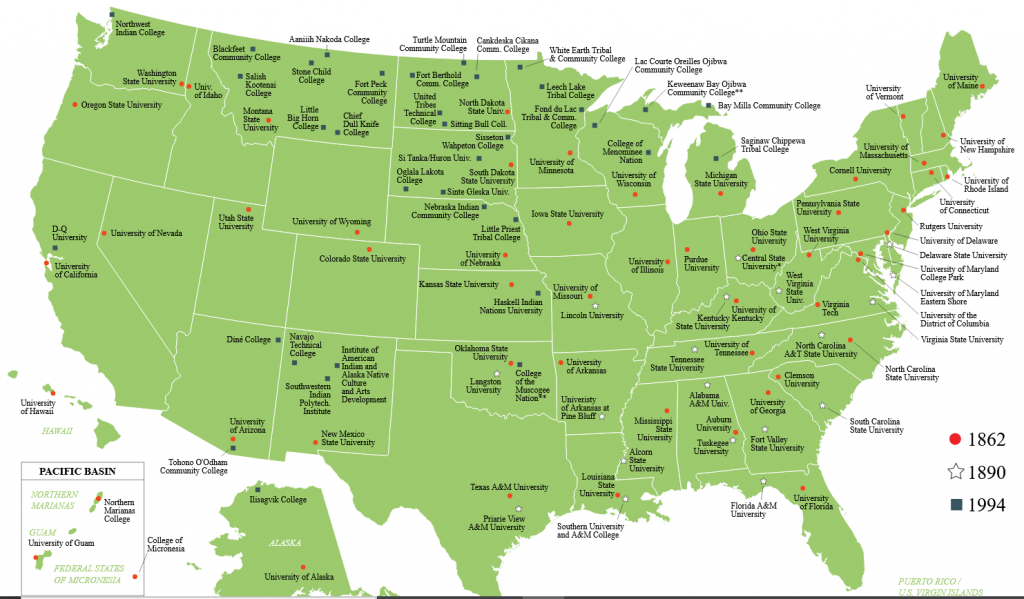
Institutions established under the Morrill Act of 1862, and its subsequent expansions in 1890 and 1994.
These institutions, more than 150 years later, are integral parts of the country we inherited. The land grant universities are engines of research, teaching, education, community outreach, and economic development in all 50 states. They created — and continue to create — a national talent pool for science, engineering, and agricultural disciplines.
This investment, more than any single initiative, set the stage for the US to build the world’s leading network of research universities. When the NIH and NSF and Defense Department research agencies hit their stride after World War II, they were positioned to send grant dollars flowing through this pre-existing network.
It was like running semi-trucks full of equipment down an Interstate highway that someone had already thought to build.
The knowledge-based industries the US dominates today – tech and biotech – can trace their origins to this series of investments.
Yes, it’s hard to be a determined optimist these days. It’s hard to think about investing big for the future in a country this divided. We have 450,000 dead in the pandemic, and more than 3,000 deaths a day. Millions more are suffering mentally, physically, economically, and spiritually. We must vaccinate about 80-90 percent of a nation of 330 million people, jump start the world’s largest economy, and get serious about creating a more fair, decent and humane society. Then we have to confront the threat of future pandemics, and get serious about climate change.
But look at what Justin Smith Morrill and his contemporaries were up against. They had to win a brutal war that left 700,000 dead, while holding the Union together, ending slavery, rewriting the Constitution with the 13th, 14th and 15th amendments, and sending in federal troops to enforce the rule of law in the former Confederate states.
Even with so many fires to put out at once, the leaders in that generation thought in expansive terms.
We are at our best when thinking along these lines. We have reason to believe it can be done, even under the hardest of circumstances.
- We won World War II, and in the process, built an enduring competitive advantage in science and technology
- The US government conceived and executed on the Apollo program during the civil unrest of the 1960s
- We used federal research funding, through DARPA, to catalyze the development of the Internet
- We spearheaded the Human Genome Project, accelerating the biotech revolution
- We invented COVID-19 vaccines from scratch, with government researchers and industry partners working together, in less than 12 months
- We have the world’s best health agencies – the NIH, FDA and CDC. These are the exemplars every other country either aspires to copy or follow. Despite recent blunders, this is still true.
For Republicans who may be skeptical of those agencies above, take a look at our national security apparatus.
For instance, in October 2013, the Defense Advanced Research Projects Agency (DARPA) made a $25 million grant to a little startup called Cambridge, Mass.-based Moderna Therapeutics. The objective was to support mRNA vaccine development for pandemic preparedness.
I interviewed CEO Stephane Bancel. At the time, I wrote:
“By making this grant, [DARPA] is betting on what could become a superfast, cheap, and unusually adaptable method for fighting today’s known pandemic threats, and the unknown threats of the future.”
The US government was prescient. Moderna wouldn’t be where it is today without DARPA and the NIH supporting it every step of the way.
So what can we do now, besides the obvious things the President and leaders in Congress are working on?
- We can triple the NIH budget over the next decade. This would be a strategic investment at a moment of biomedical possibility, and it would be done at a scale that is beyond the scope of any entity other than the US government.
- We can shake up the FDA. It needs to be restored as the world’s best public health regulatory agency, one that thoughtfully facilitates the creation of safe and effective medicines that unleash human productivity. Here, the industry has a clear voice through renegotiating the Prescription Drug User Fee Act (PDUFA), that comes due in September 2022. Let’s re-think regulatory frameworks for things like master protocols and adaptive designs. Let’s invest in studying technology-enabled biomarkers from real-world experience of patients who carry smartphones, as part of a push to reinvent the R&D enterprise with leaner and meaner clinical trials. If we’re willing to spend some money, and able to attract the right leadership, we’ll create an FDA that can operate at the speed and scale necessary to keep up with industry, and keep up with health needs.
- We can build up our community colleges with programs in advanced biologics manufacturing, where there’s a need for domestic skilled labor. If we’re serious about filling a pipeline with people who seize these opportunities, we’ll bolster our support for K-12 public schools.
Our failure to invest in young people is mind-boggling.
Consider a 2017 report from the Pentagon. It says that 71 percent of Americans ages 18-24 are ineligible to serve in the US military primarily because of three reasons: obesity, failure to graduate from high school, or a criminal record.
“This is a very real risk to our national security,” said Steve Doster, Pennsylvania State director of Military Readiness for Council for a Strong America, told the York Daily Record in 2019.
It’s also a sign of a country struggling with apathy. Our young people are yearning for purpose and hope. We’ve seen in other countries what happens when young people are hopeless. They turn to violent extremism, or surrender, living in fear of authoritarian leaders.
Biotech, as I wrote here last month, is shining with possibility amid the darkness. It’s one of the bright spots in the US. It’s why I think this industry needs to shine that light as far and wide as possible.
Those of you in industry can share the wonder and joy of discovery, especially with underserved communities that are full of untapped human potential. If kids in middle school and high school knew a fraction of what’s happening in biomedicine today, and they could pursue advanced study without taking on a mountain of debt, they’d tune in. (Life Science Cares is a terrific organization that connects biotech companies with nonprofit partners that advance education, and anti-poverty work.)
Too many people have given up on the American dream. We need to shake off the malaise. We should know our history, and never forget where our amazing scientific enterprise comes from.
Science of SARS-CoV-2
- Single Dose Administration, And The Influence Of The Timing Of The Booster Dose On Immunogenicity and Efficacy Of ChAdOx1 nCoV-19 (AZD1222) Vaccine. Preprints with The Lancet. Feb. 1. (Merryn Voysey et al University of Oxford – Oxford Vaccine Group)
- Learning the Language of Viral Evolution and Escape. Science. Jan. 15. (Brian Hie et al at MIT)
- SARS-CoV-2 Infects and Replicates in Cells of the Human Endocrine and Exocrine Pancreas. Nature Metabolism. Feb. 3. (Janis Muller et al)
- Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. The Lancet. Feb. 2. (Denis Logunov et al)
- Age groups that sustain resurging COVID-19 epidemics in the United States. Science. Feb. 2. (Melodie Monod et al Imperial College London)
- Genomic monitoring of SARS-CoV-2 uncovers an Nsp1 deletion variant that modulates type I interferon response. Cell Host and Microbe. Jan. 29. (Jing-wen Lin et al)
Vaccines
- Trump officials actively lobbied to deny states money for vaccine rollout last fall. STAT. Jan. 31. (Nicholas Florko)
- Covid-19 Vaccine Makers Take Aim at Dangerous New Strains. WSJ. Feb. 3. (Peter Loftus and Jared Hopkins)
- With a Seductive Number, AstraZeneca Study Fueled Hopes That Eclipsed its Data. STAT. Feb. 3. (Matthew Herper and Helen Branswell)
- Novavax Vaccine Works Well, Except on Variant First Found in South Africa. NYT. Feb. 3. (Katie Thomas, Carl Zimmer and Sharon LaFraniere)
- Johnson & Johnson Single-Shot COVID-19 Vaccine Met Primary Endpoint in Interim Analysis of Phase III Ensemble Study. (J&J press release).
- J&J seeks FDA EUA. (J&J press release).
- J&J and Novavax Data. In the Pipeline. Jan. 29. (Derek Lowe)
- The EU approves AstraZeneca’s Vaccine and Moves to Restrict It From Sending Doses Outside the Bloc. NYT. Jan. 29. (Matina Stevis-Gridneff)
Access and Distribution
- Covid-19 Vaccines Leave Pregnant Women in a Quandary. WSJ. Feb. 1. (Sarah Toy and Laura Cooper)
- COVID-19 Vaccines to Stress Test Grocery Stores and Pharmacies. WSJ. Feb. 2. (Sharon Terlep and Jaewon Kang)
- Biden Covid Team Derides Trump Plan While Borrowing Playbook. Bloomberg News. Jan. 28. (Josh Wingrove and Riley Griffin)
Genomic Surveillance
- Why America Is Flying Blind to the Coronavirus Mutations Racing Across the Globe. Washington Post. Jan. 29. (William Wan and Ben Guarino)
- COVID won’t be last pathogen to threaten our national security. We need to prepare better. USA Today. Feb. 4. (Dylan George and Caitlin Rivers)
- What’s Going on With All Those Variants? An Illustrated Guide. NPR Goats and Soda. Feb. 2. (Michaeleen Doucleff and Meredith Rizzo)
Features
- As Scientists Turn Attention to COVID-19, Other Research Isn’t Getting Done – And It Could Have Lasting Consequences. The Conversation. Jan. 29. (Julie Pfeiffer of UT Southwestern and Terence Dermody of University of Pittsburgh)
- In the Vaccine Scramble, Cancer Patients Are Left Behind. NYT. Feb. 3. (Dani Blum)
- COVID is Here to Stay. Interview with Pfizer CEO Albert Bourla. Bloomberg News. Jan. 28. (John Micklethwait)
- Refreshing the Biologic Pipeline 2020. Nature Biotechnology. Jan. 28. (John Hodgson)
Personnel File
Merck CEO Ken Frazier announced he plans to retire June 30, 2021 after a decade in the job. He will continue as executive chairman. Robert Davis, the current CFO, will become president in April and CEO on July 1 as part of the transition process. (See Matt Herper’s STAT article on Frazier’s career).
Merck said Stephen Mayo, a professor of biology and chemistry at Caltech, is joining the board of directors Mar. 15.
Ramona Sequeira, president of Takeda Pharmaceuticals USA, is the new chair-elect of PhRMA. She will be the first woman to serve as chair of PhRMA. She will replace Eli Lilly CEO Dave Ricks in that role next year. Novartis CEO Vas Narasimhan was elected to serve as PhRMA board treasurer.
Germany-based Merck KGaA said Rehan Verjee, the president of EMD Serono and head of innovative medicines, is leaving the company for a new job to be announced. He’s being replaced by Andrew Paterson.
South San Francisco-based Twist Bioscience, the DNA synthesis company, announced a series of promotions. Siyuan Chen was promoted to chief technology officer and Aaron Sato was promoted to chief scientific officer. Paula Green was promoted to senior vice president of human resources, and Angela Bitting joined the company as senior vice president of corporate affairs.
Cambridge, Mass.-based Scholar Rock announced a string of promotions, including Gregory Carven’s promotion to chief scientific officer.
Cambridge, Mass.-based Revitope Oncology, a cancer immunotherapy company, added Louis Lange to its board of directors. He’s a general partner at Asset Management.
Cambridge, Mass.-based TCR2 Therapeutics named Shawn Tomasello to its board. She has a 35-year track record in commercial operations and medical affairs, including time at Pharmacyclics, Celgene and Genentech.
Cambridge, Mass.-based Moma Therapeutics named Asit Parikh as its new CEO. It’s a precision medicine company backed by Third Rock Ventures.
Burlingame, Calif.-based Lyra Health, the provider of mental health care benefits for employers, added Danielle Gray to its board of directors. She is senior vice president, chief legal and administrative officer for Blue Cross and Blue Shield of North Carolina.
Katie Porter’s Swing-and-a-Miss
Rep. Katie Porter of California is a smart progressive and a formidable prosecutor. I cheered when she grilled former Celgene CEO Mark Alles about that company’s unjustifiable price increases for Revlimid. He deserved a public dressing down after getting wealthy while running the company into the ground. Rep. Porter is channeling much of the frustration people have with our current form of capitalism, which punishes people who follow the rules and rewards the lying, cheating and stealing.
But Rep. Porter issued a 16-page report on mergers and acquisitions in biopharma late last week that was an oversimplified rant that won’t do anyone any good. She called it a “bombshell” report on how M&A stifles innovation.
This report is actually a swing-and-a-miss.
Porter used the Amgen-Immunex acquisition of 2002 as a case study. I covered the Amgen-Immunex deal day-by-day for The Seattle Times. It was a fascinating, nuanced story. Porter’s team interviewed a number of former Immunex employees. They rightly complained about how the vibrant scientific culture at Immunex got smothered. But Porter missed some crucial context to understand the situation. She failed to mention that Immunex screwed up manufacturing. It couldn’t make enough etanercept (Enbrel) to meet the demand from rheumatoid arthritis patients. They left tens of thousands of patients in the lurch, struggling in pain. That fiasco depressed its stock, making it vulnerable to takeover.
Amgen, arrogant and stifling as it was in many ways under CEO Kevin Sharer, was able to fix the manufacturing supply problem in short order. Patients were better served by having Enbrel in its hands. And Amgen, over time, was able to combine some IP with Immunex that led to the development of denosumab (Prolia) for osteoporosis and (Xgeva) for bone metastases.
In the big picture, is M&A good for innovation? I’m no fan of megamergers like BMS-Celgene, because they create organizations so big it’s hard for anyone to do nimble, innovative work. But most deals aren’t like that. Often, but not always, a new product is better off in the hands of a big company. Small companies and their investors also need to know there’s a strong incentive, a pot of gold at the end of the rainbow, if they’re going to invest and do the risky, innovative work in the first place. As John LaMattina rightly pointed out in Forbes, the Pfizer / BioNTech partnership in mRNA vaccines is a great example of different kinds of players playing to their respective strengths. Neither the big company or the small company could have made the vaccine alone. It took both entities, working together, to pull off that historic feat.
The Federal Trade Commission needs to have clear and consistent criteria for when to bring antitrust cases, like when a merger is really about snuffing out competition or depressing innovation to protect an existing franchise. FTC prosecutors need to exercise good judgment on when to take action. Members of Congress should provide oversight and accountability.
Rep. Porter, instead of painting in broad brushstrokes about the ills of M&A, would be wise to leave this work to the FTC. We don’t need more complex issues like this reduced into dumbed-down left-right food fights. That sort of thinking has done a lot of damage already.
Deals
Jazz Pharmaceuticals agreed to pay $7.2 billion to acquire London-based GW Pharmaceuticals, the marketer of cannabidiol oral solution (Epidiolex) for epileptic seizures. The deal includes $200 a share in cash and $20 in Jazz ordinary shares.
Gilead Sciences agreed to work with Gritstone Oncology to use the smaller company’s vaccine technology to work on an HIV cure. The plan is to develop a prime-boost product “comprised of self-amplifying mRNA (SAM) and adenoviral vectors, with antigens developed by Gilead.” Gritstone is getting $30 million in cash and $30 million in an equity investment from Gilead.
South San Francisco-based Veracyte agreed to acquire San Diego-based Deciphera Biosciences for $600 million — $250 million in cash and up to $350 million in stock. The deal strengthens Veracyte’s position in genomic diagnostics for cancer, and provides a foothold in another biotech talent pool.
GSK and Germany-based CureVac agreed to work together on multi-valent mRNA vaccines for COVID-19, which hopefully will be able to address multiple variants in a single vaccine. The deal is worth 150 million Euros, and GSK said it will support manufacturing of 100 million doses in 2021.
Horizon Therapeutics agreed to acquire Gaithersburg, Maryland-based Viela Bio for $3.05 billion or $53 a share.
Bristol-Myers Squibb agreed to in-license a COVID-19 treatment composed of two monoclonal antibodies from Rockefeller University. The dual antibody treatment is designed as a longer-lasting subcutaneous formulation, that is supposed to be more suitable to give to non-hospitalized patients than earlier antibodies that are given intravenously.
Redwood City, Calif.-based Coherus Biosciences paid $150 million upfront to Shanghai Junshi Biosciences to obtain US and Canada rights to a late-stage PD-1 inhibitor, toripalimab. Coherus also obtained an option to Junshi’s TIGIT and IL-2 drug candidates that could be used in combination.
A group of more than a dozen pharma R&D leaders have formed a new COVID-19 R&D alliance.
Financings
ARCH Venture Partners raised a new fund with $1.85 billion to create and finance new biotech startups. ARCH listed a long list of interests for this new fund — infectious disease, mental health, immunology, oncology, neurology, manufacturing, clinical trials, anti-aging medicines, genomic and biological tools, data sciences, and ways of reimagining diagnostics and therapies.
Abingworth raised its 13th biotech fund, Abingworth Bioventures 8, with $465 million to invest. It has already made three investments in the new fund, including one in gene therapy company Atsena (profiled in TR, December 2020)
Seattle-based Sana Biotechnology raised $588 million in an IPO of 23.5 million shares at $25 a share. Initial valuation: $4.9 billion. The cell therapy company is still in preclinical development. It’s the largest preclinical stage IPO ever. Shares climbed another 40 percent in first-day trading to close at $35.10.
Boston and Rockville, Maryland-based Sensei Biotherapeutics, a cancer drug developer, raised $133 million in an IPO at $19 a share.
Watertown, Mass.-based EyePoint Pharmaceuticals raised $100 million in an IPO at $11 a share. The company is developing a treatment for the wet form of age-related macular degeneration.
23andMe, the consumer genetics company, went public through a SPAC sponsored by Richard Branson’s Virgin Group. The deal comes with $759 million of proceeds ($509 million in cash, plus a $250 million private placement), and pegs the company’s enterprise value at $3.5 billion.
Blacksburg, Virginia-based Landos Biopharma raised $100 million in an IPO priced at $16 a share. It’s working on treatments that work on novel mechanisms for autoimmune diseases. The lead candidate is for ulcerative colitis and Crohn’s disease, and targets the LANCL2 pathway.
Biogen said it’s issuing $1.75 billion in debt.
Billerica, Mass.-based Quanterix, which does digital biomarker analysis for precision health, raised $200 million in a stock offering.
Redwood City, Calif.-based Revolution Medicines raised $261 million in a stock offering at $45 a share.
Lexington, Mass.-based Kaleido Biosciences raised $60 million in a stock offering at $11.50 a share.
Regulatory Action
Biogen and Eisai said the FDA has extended its review of the Alzheimer’s drug aducanumab by another three months, to a review deadline of June 7.
The FDA cleared tepotinib (Tepmetko) from Merck KGaA as a new treatment for metastatic non-small cell lung cancer (NSCLC) harboring mesenchymal-epithelial transition (MET) exon 14 skipping alterations.
RIP
Dr. Emil Freireich, a pioneer of treatments for children with leukemia, died at age 93. (USA Today obituary)
Andrew Brooks, the Rutgers scientist who developed a quick saliva-based test for COVID-19 in the early days of the pandemic, died of a heart attack at 51.





