Get In-depth Biotech Coverage with Timmerman Report.
6
Jan
2022
The Omicron Wave: A Review of the Research on Spread and Severity

Mara Aspinall, managing director, BlueStone Venture Partners; professor of the practice, biomedical diagnostics, Arizona State University; advisor, Rockefeller Foundation
The vast majority of people in the US have at least partial immunity to many forms of SARS-CoV-2, either from prior infection, vaccination, or both.
The past few weeks have shown us that the Omicron variant transmits faster than any of its predecessors. That essentially guarantees that the unvaccinated plus early vaccinees (last dose/boost more than 6 months ago) will contract COVID-19 over the next few weeks and months.
Early reports from hospitals are telling us that people with Omicron infections are suffering less severe symptoms. If this phenomenon — the combination of airborne transmissibility and mild lower airway symptoms holds up over time — then it is likely that COVID-19 will join the common cold, Influenza and Respiratory Syncytial Virus (RSV) among endemic infectious diseases.
We aren’t going to eradicate COVID. We’re going to have to live with it.
Let’s consider a couple key questions in some depth:
- What evidence is there that we are entering a COVID endemic era in 2022?
- What will a COVID endemic world look like (especially for diagnostics)?
1. What evidence is there that we are entering a COVID endemic era?
Omicron has become the dominant variant of concern in an even shorter time than Delta, the previous record holder. Because of its plethora of mutations on the Spike protein, Omicron has effective resistance to most previously developed antibody drug cocktails and substantially reduces vaccine effectiveness.
On the good news front, Omicron is causing far fewer hospitalized, ICU or ventilated patients per infection. Laboratory work suggests several compelling hypotheses of why this might be so. Our confidence is mounting that Omicron has higher transmission but lower morbidity and mortality than prior variants of concern: perhaps to the level of seasonal flu.
However encouraging that may be, influenza fatality rates can be tragically high (e.g. 61,000 deaths in 2017-18 in the US). A world with both flu and COVID circulating at the same time will inevitably increase respiratory distress mortality and morbidity, and put tremendous pressure on hospitals.
Is there real-world evidence of lower morbidity and mortality? Early indications: Yes
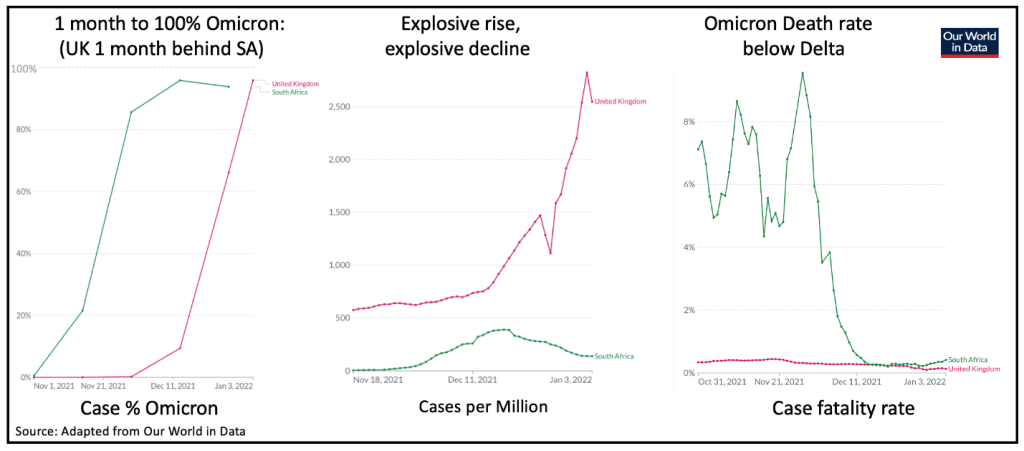
The best data on Omicron comes from South Africa and the UK, where Omicron developed the earliest and where surveillance has been the most comprehensive. The UK is running about 4 weeks behind South Africa, where the drop in Omicron’s death rate was particularly striking.
The UK pre-existing Delta death rate was already much lower than South Africa, but that too has more than halved in the Omicron era.
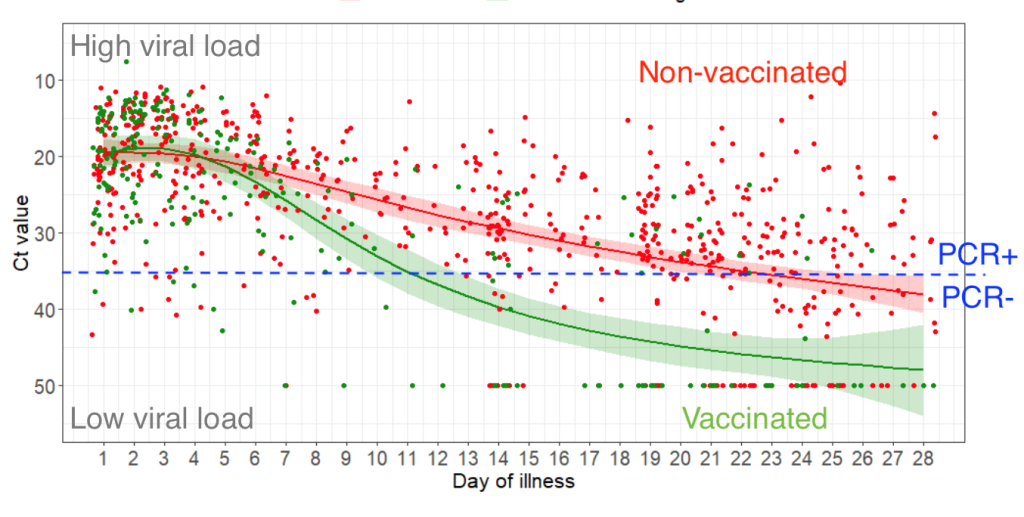
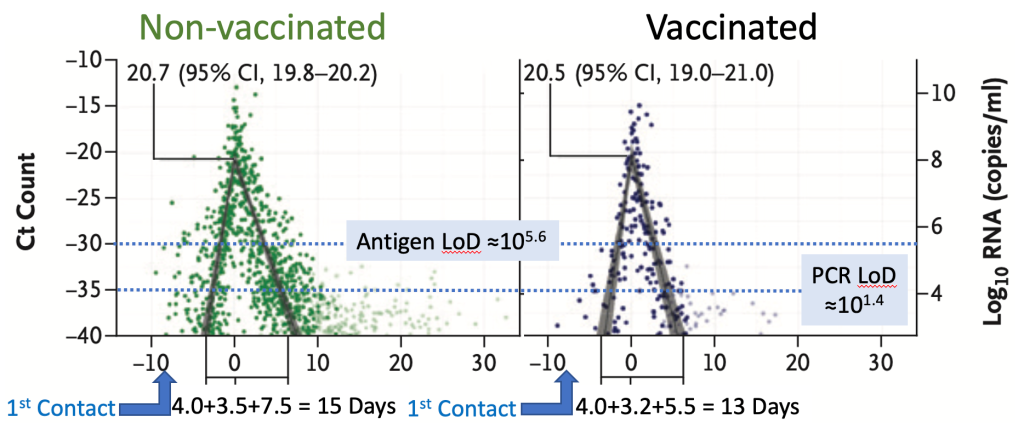
The rate of asymptomatic Omicron cases is much higher than prior variants, according to a Dec. 27 study from South African researchers and colleagues at the Fred Hutchinson Cancer Research Center. Researchers found that almost one-third (31 percent) of people with HIV who enrolled in a Moderna vaccine study in South Africa in December were found to already have COVID. That’s up from about 1-2 percent of people who showed up in a comparable vaccine study months ago, when the Beta variant was circulating. These asymptomatic carriers were shown to have high levels of viral shedding based on samples from nasal swabs.
This high rate of hidden cases, and the high levels of viral shedding, means that many people will inevitably transmit their infections to others. Some of these people will end up with severe symptoms.
That’s a glimpse at the biology. Now let’s take a look at the clinical implications.
Researchers at Imperial College London looked at data from the first two weeks of December, when Omicron rose to prominence. The team reported 40-45% lower hospital admissions for Omicron versus Delta (Table 1: note UK “hospitalization” includes emergency department visits, admissions is more representative of severity). Prior infection and vaccination reduced the risk of Omicron hospital admission risk by ~70% (Table 2).
By the end of December, with more data coming in every day, the hospital admission rate in London was about 80 percent lower during this Omicron wave than the prior Delta wave.
How are the vaccines holding up?
The UK Health Security Agency has published an update on vaccine effectiveness after 198,348 UK Omicron cases (Omicron update 12/31/2021).
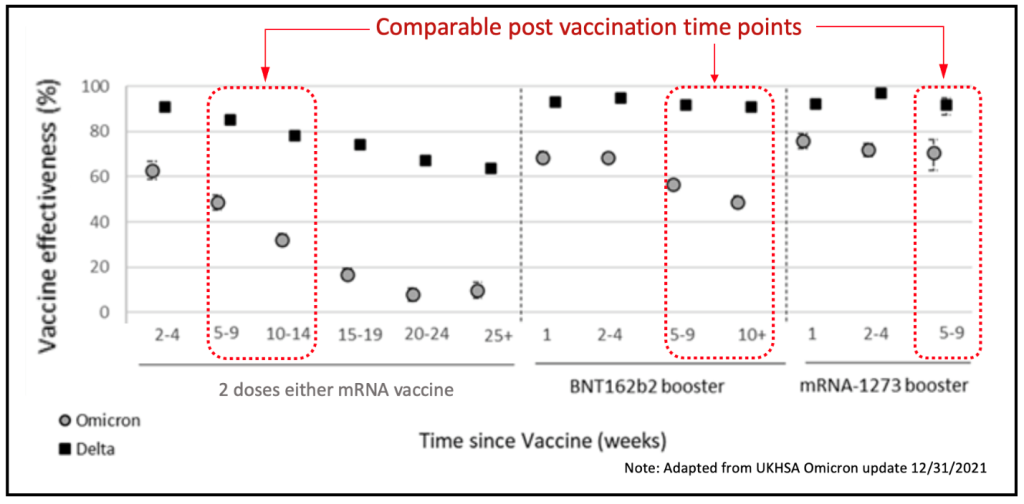
Bottom-line, a two-dose mRNA vaccination series confers essentially no protection against Omicron infection after 6 months, whereas the two-dose mRNA vaccine retained ~60% effectiveness against Delta (down from 98% initially). A subsequent booster increases Omicron immunity at the 9-10 week point from a rapidly declining ~40% back up to ~50% (Pfizer boost), or ~70% (Moderna boost).
There has not yet been enough time to evaluate longer term effectiveness versus Omicron.
Prior infection, current vaccines and boosters do reduce severity, but cannot be considered effective against infection beyond 6 months. The saving grace of the vaccines, however, can be seen in the hospitalization data referenced above. They are keeping people out of the hospitals.
There hasn’t been enough time yet to fully evaluate the US clinical experience, at least compared with the UK and South Africa. But all the signs are that it will be consistent here (for an always helpful US perspective, see Paul Sax MD’s blog of Dec. 30)
Evidence from the Laboratory to Explain Reduced Disease Severity
Over the past month, there have been many excellent studies (I have 16 on file currently and more arrive every day) examining the pathology of Omicron infection in animal models and human cell cultures. There are two arenas contributing to a consensus on why Omicron is demonstrating lower morbidity and mortality (see Eric Topol’s excellent “Ground Truths” newsletter on Substack):
Omicron infects primarily the upper respiratory tract – especially the nose and throat. That’s likely causing more coughing and viral aerosol dispersion and transmission. The virus appears to be less able to migrate further the lower respiratory tract, deep into the lungs where prior variants dramatically damage the cells – alveoli – responsible for clearing carbon dioxide and transferring essential oxygen to the bloodstream. This is an evolutionarily favorable path for viral mutation, as evidenced by Omicron’s competitive advantage over Delta, which is more efficient at getting deep into the lungs. This does not preclude a more virulent future path for Omicron, but it does reduce its likelihood.
See the following studies below for more detail:
-
- Lungs have 1,000x lower viral load and no damage (12/26/21 animal model)
- Omicron 70x higher virus in Bronchus; 10x less in lung (12/15/21 human tissue)
- Omicron viral load and damage lower in lung (12/29/21 human tissue)
- Cell fusion reduces white cell effectiveness, only evident in severe cases (5/12/21 Commentary/Review)
- Damaging cell fusion (syncytia) are common in viral diseases (9/21/21 math model)
- Omicron causes lower cell fusion closely related to pathogenicity ( animal model)
- Lower lung absence of cleavage protein (TMPRSS) inhibits lung infection (12/22/21 cellular model)
- Less lung infection and inflammation from Omicron (12/28/21 humanized mouse model)
- Milder infection in all tissues, limited pathology (12/28/21 animal model)
- Broader set of tissues infected, but less in the lungs (1/3/22 human cells)
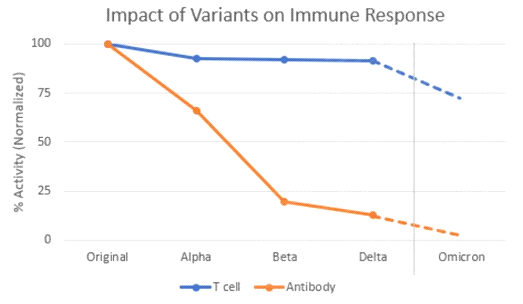
Although neutralizing antibody counts from prior infection or vaccination do decline toward zero after 6 months, memory B cells (the cells that produce these antibodies) and memory T cells (that mobilize the immune system and attack pathogens directly) both become more broadly effective against unencountered variants after repeated prior exposures.
Generally speaking: the more exposure the better, especially with longer delays between encounters (implies minimum 12-16 week spacing of each initial and booster dose). One of the key early findings of the Omicron wave is that T-cell immunity from prior infection and vaccination appears to be longer-lasting, and this second-line of immune defense is likely a big reason why so many people are getting mild infections that don’t end up sending them to the hospital.
See a few key studies that look beyond neutralizing antibody assays below:
-
- Longer infection/vaccination intervals increase resistance (1/1/22 multi-country)
- Enhanced memory B cell effectiveness on repeated exposure (1/1/22 patient cells)
- 70-80% of T cell effectiveness is retained across all variants (12/28/21 patient samples)
- Omicron infection provides cross-immunity to prior variants (12/27/21 patient samples)
- Broad immune escape but little lung damage (1/3/22 cells and epidemiology)
2. What will a COVID endemic world look like (especially for diagnostics)?
First, let’s look at this in the short term to mitigate the damage from an ongoing airborne threat.
- In the US, we need to persist in efforts to vaccinate the remaining 40% of the population that remains unvaccinated (including children). The vaccination push should require boosters at 6 months. Efforts should continue to revise vaccines for novel-variants and conserved epitopes. If successful, these updated vaccines could be available by late 2022/early 2023
- Continue masking in, and/or avoidance of, enclosed spaces
- Continue and systematize surveillance through viral genomic sequencing (and PCR)
- Empower individuals and institutions with widely available and inexpensive self-tests
There has been massive demand in the US for rapid testing. Individuals have become more familiar with self-testing. This is not about to decline. The industry needs to develop expanded availability of rapid tests to allow self-diagnosis to distinguish among the broad range of respiratory diseases – tests that can discriminate among both bacterial and viral respiratory infections – COVID-19, Influenza, RSV, tuberculosis, pneumonias etc.
Without adequate testing and surveillance, it’s hard to answer a key question — how will we know if it is “over” (at least in its current form)?
Official counts of cases are becoming less reliable. Few of the rapid self-test results are reported to public health authorities who manage the official databases. Omicron’s increased asymptomatic cases will largely go untested — people will have COVID and walk around infecting other people without ever knowing they were positive.
In this context, reported case numbers understate the reality, even as the total number is rising exponentially. Given this reality, some have suggested we should primarily track hospital admissions since this is generally a reliable indicator of serious disease (although COVID deniers are advocating ignoring the hospitalized with COVID and only counting the hospitalized for COVID).
Unfortunately, we now know that viral loads and hence transmission can be just as high in the asymptomatic – we cannot afford to lose track of the total number of cases.
A continuing unknown is the long-term effect of past infection. We do know that virus is found in many organs on autopsy; a trend likely exacerbated by Omicron as a result of its reduced ACE2/TMPRSS dependency. A 1/2/2022 University of Waterloo Canadian paper suggests that executive function is significantly affected during post-acute COVID-19 recovery, but we do not yet know what circumstances drive vulnerability to Long COVID-19, nor what types of infection cause it.
This is one of the big research questions that will take time to answer – to what extent does Omicron infection lead to various forms of Long COVID? What biological phenomena are driving the chronic symptoms? How many people will be affected, and for how long? What kind of symptoms will they have to endure?
It will take time to gather data to help us understand what’s going on.
It has been 100 years since the devastating 1918-1921 Influenza pandemic. That avian H1N1 virus infected one-third of the global population, with nearly 5% mortality among younger adults. We have been fortunate that COVID-19 has had a relatively low mortality rate of 1-2%, but it is clear that 21st century travel and COVID-19 show that infection can spread quickly to more than just one third of the global population – by the time this pandemic is over, 70-80% of the global population will have been exposed, infected and/or vaccinated.
We have been negligent preparing for inevitable waves of epidemic infections. We have few effective viral therapies in hand. We are rapidly exhausting the antibiotics that have protected us from bacteria scourges for the past 70 years.
Given that it is highly unlikely that COVID-19 disappears, we are beginning the transition to an influenza-like endemic COVID: a continuing threat of more virulent mutation-fueled outbreaks driving ever-present danger to the respiratory impaired and those with compromised immunity.
The last 50 years have taught us that animal reservoir pathogens can mutate and spread without warning — we cannot allow ourselves to fall under the spell of complacency ever again.