Get In-depth Biotech Coverage with Timmerman Report.
17
Dec
2020
Round 2: What’s Changed and What Hasn’t in Caring for Outpatients with COVID-19

Alex Harding, MD. Entrepreneur in Residence, Atlas Venture
When one of my COVID patients called my cell phone a few weeks ago, my stomach dropped.
“I feel worse,” she stammered. “I’m having more trouble catching my breath.”
It was late morning on a Sunday. I was planning to take my son to the park, but after hearing those words, I knew this was not going to be a quick phone call.
I had seen the patient, a woman in her late 30s, the previous afternoon in the respiratory illness clinic at Massachusetts General Hospital (MGH), where I’ve worked since the Biogen outbreak slashed through Boston in early March.
She had typical COVID symptoms: fever, muscle aches, chest tightness, and mild shortness of breath when she walked around her house. She was obese and prediabetic, but otherwise healthy. When, sure enough, her COVID test returned positive the next day, I called her hoping that she would already be feeling better.
Instead, what ensued was a conversation that my colleagues and I have had far too often in the past several weeks.
“What can I do to get better?” she asked.
The fear in her voice was unmistakable, as she could feel herself losing strength. Stories of critically ill COVID patients suddenly seemed much more personal. I’ve seen more than 100 COVID patients this year, and the sheer variability of their responses has been befuddling. I had no obvious answer at my fingertips.
I rubbed my brow as I contemplated a response. What treatments could I offer her? Was there anything that might stop her illness from worsening?
Should I tell her to go to the emergency department now?
To a doctor on the front lines, the first surge felt like standing knee-deep in surf, looking out at a choppy sea and seeing a tidal wave on the horizon. It was approaching rapidly, but I couldn’t see to the top of it to know how hard it would hit or when it would end. There were people bobbing in the water, and somehow, I had been designated as a lifeguard. And I didn’t even know how to swim.
This, the second surge here in Massachusetts, feels different. It’s a similar tidal wave, but I have an idea how tall it is and how hard it will hit. I’ve learned how to swim. But I’m still swimming unassisted—no life preserver, no flares, no rescue boat. Today, we know what COVID looks like.
We’ve learned a lot about the management of severely ill patients in the hospital. Pronation can help hospitalized patients breathe better. Dexamethasone can help the most severely ill patients and there’s a place for remdesivir in severely ill hospitalized patients as well. But we are still staring up at tidal waves of the unknown, particularly for non-hospitalized patients.
We still don’t know which outpatients will do well and which ones won’t; we still don’t know the best management strategies for common clinical situations; and we still don’t have any meaningful therapies to offer our patients outside of the hospital.
Remdesivir and dexamethasone have received a lot of attention for their utility in treating COVID, but they are only useful for patients inside the hospital. Remdesivir is an intravenous therapy and has not demonstrated efficacy in outpatients. Dexamethasone is only authorized for hospitalized patients on supplemental oxygen, and may actually worsen disease in patients with less severe disease.
The multiple vaccines with outstanding data, of course, offer hope for limiting new cases of the virus starting this spring. But they do nothing for people who are already infected, people who are suffering now and expected to continue filling up hospital beds in days and weeks ahead.
The FDA has recently authorized two medications for patients outside the hospital, Eli Lilly’s bamlanivimab and Regeneron’s casirivimab/imdevimab. Neither of them appears to be a transformative treatment for outpatients with COVID.
Bamlanivimab is a monoclonal antibody developed by Eli Lilly that is running a clinical trial in 452 outpatients with mild or moderate COVID. My clinic at MGH is one of the sites on that clinical trial, and I have referred patients to participate. The drug was authorized by the FDA on the basis of an interim data analysis that showed a clinically modest, but statistically significant, reduction in viral load at the middle of three doses. At that mid-level dose, the average viral load was reduced by 99.99% by day 11 of the study.
Sounds great, right? The catch is that, across all groups, including placebo, the average viral load was reduced by more than 99.9% by Day 11. In fact, the high dose and the low dose were statistically no different than placebo. The high dose, puzzlingly, actually had a lower percent reduction than placebo, although it was not a statistically significant difference.
It’s important to know that a treatment designed to neutralize the virus actually does bring down viral loads. Then again, doctors and patients do not care about a difference in viral load of less than 0.1% in and of itself. ‘Harder’ clinical endpoints like hospitalization, intubation, and death are much more meaningful. The interim readout of the trial – the basis for the FDA’s Emergency Use Authorization (EUA) — did not include enough hospitalizations to make firm conclusions on this endpoint. There were five hospitalizations (1.6%) among all three treatment doses—including the doses that did not reduce viral load—and nine hospitalizations (6.3%) in the placebo group.
In summary, there was a reduction in viral load in the middle dose that you might be able to see if you squint, and insufficient evidence to draw any conclusions about hospitalizations. It is a weak body of evidence to support a drug authorization—a strain to justify, even during a pandemic.
But the authorization was not just a strain. It was bizarre. Instead of authorizing the middle dose—the only dose that actually showed a reduction in viral load—the FDA authorized the low dose, which showed no benefit in viral load. The FDA’s explanation was that there would be more drug to go around if each patient got the lower dose. Yet every doctor I know would prefer to treat one patient effectively than four patients ineffectively.
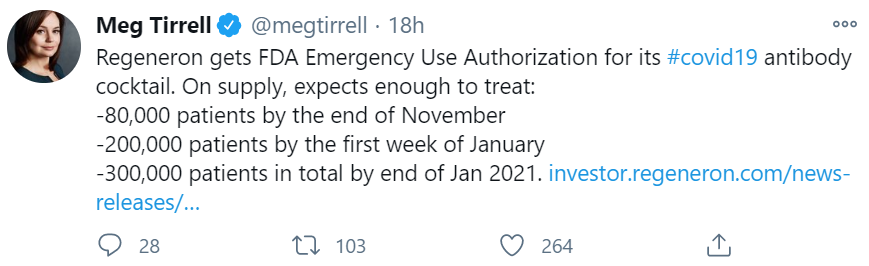
The other treatment that the FDA recently authorized for outpatients is casirivimab/imdevimab, Regeneron’s dual antibody cocktail. The data for this combination looks similar to the bamlanivimab data. The reduction in viral load was modest but statistically significant for both doses tested. There were fewer hospitalizations and emergency department visits in the treatment groups than the placebo group, although the number of events was again too low to draw any conclusions.
The good news with this treatment is that the FDA authorized a dose that appears to actually do something (since both doses showed viral load reduction). The bad news is that the complete data from the clinical trial has still not been published, over three weeks after the authorization went into effect. The FDA included snippets of data in its EUA, but Regeneron has still not published a complete dataset in a peer-reviewed journal, or even on a pre-print database. As such, it is impossible for me to have an informed opinion on the utility of this drug for my patients.
Further complicating the muddy data for bamlanivimab and casirivimab/imdevimab is the logistical complexity of administering these treatments. Both are intravenous (IV) infusions. For outpatients, IV infusions are usually administered in an infusion center, a dedicated clinical unit. However, since these patients are infected with COVID, they cannot mix with other patients, requiring the creation of entirely new infusion spaces specifically for this purpose.
At MGH, a hospital fortunate to have the resources to create such a space, a central team reviews every patient case and determines who would be eligible for treatment, then places them in a lottery to see who can get the treatment. I don’t even have control over the prescribing process for my own patients.
We lack more than just medicines to treat outpatients with COVID. Many of the basics of clinical management of these patients remain undefined. We know that most patients with COVID have only mild disease, but a significant minority of patients develop severe, life-threatening illness. One of my biggest challenges is identifying the patients who are at risk for rapid clinical worsening. Several research groups are investigating tools to risk stratify patients, but none seems to be a definitive solution so far.
My colleagues and I are running a prospective trial in our clinic to see if exertional oxygen saturation (a patient’s blood oxygen level during light exercise) can identify the patients who are at risk of getting worse. Other groups have used machine learning to pull clues from patients’ charts. Chest X-rays and blood tests might be helpful. This is a huge clinical problem and I hope that one of these approaches turns out to be a reliable predictor of outcomes, so we can act accordingly to help patients early in their course of disease.
Management of a plethora of other clinical situations in outpatients with COVID also remain uncertain. Here are a few examples:
- Should patients with confirmed COVID and infiltrates on chest X-ray (i.e., pneumonia) be prescribed antibiotics for a possible secondary bacterial infection? If so, what antibiotics and for how long?
- Should patients with COVID who also have asthma be prescribed oral steroids, or should these be avoided when possible given potential harm of dexamethasone in mild-moderate COVID?
- How should chest pain be evaluated in a patient with COVID and low/moderate cardiovascular risk factors?
- How should longer term effects of COVID be managed? What is appropriate counseling regarding these longer-term effects?
These questions come up nearly every day I am in clinic, yet there are no clear guidelines for handling any of these situations. There is an enormous amount about this disease that we still don’t know.
On top of better guidance on those clinical questions, we are in dire need of better therapies for outpatients. I am grateful for the antibody therapies that are being developed, but what we really need are potent, oral antiviral therapies. These drugs are probably a year or more away, because medicinal chemistry efforts to discover novel compounds with desirable pharmaceutical properties are necessarily slow. Repurposing existing compounds, as was done with remdesivir, is unlikely to be the solution because these compounds rarely have the potency and desired properties to make for a truly transformative therapy.
Let’s return to my phone conversation with my patient with worsening COVID.
“What can I do to get better?”
What could I say? I seriously considered telling her to go to the emergency department right then, but because she was young and in satisfactory health at baseline, I decided against it. I encouraged her to sign up for the clinical trial of bamlanivimab—that way, she would at least have a chance of getting the dose that reduces viral load. And I told her that if she started to feel any worse at all, she should call an ambulance.
I kept a close eye on her chart over the next week. She did enroll in the bamlanivimab trial, although I’ll never know which dose she received, or if she got placebo. In any case, she did not need to be admitted to the hospital and has since recovered from her illness. Her case was a small victory in a season full of losses. While I take pleasure in her recovery, I probably can’t take credit for it.
Not without better tools at my disposal to manage patients like her.