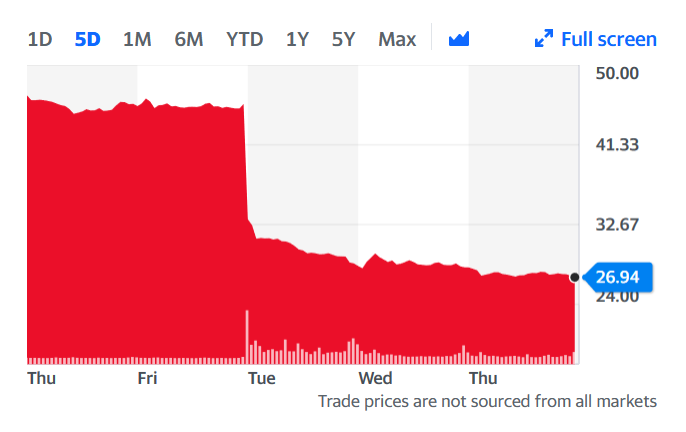
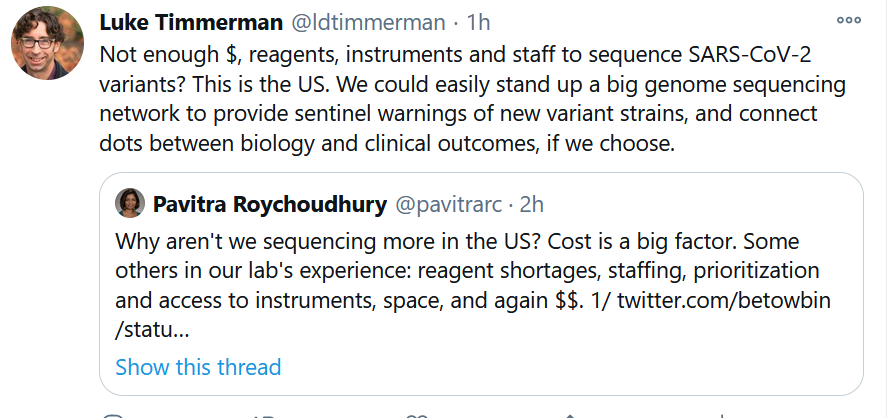
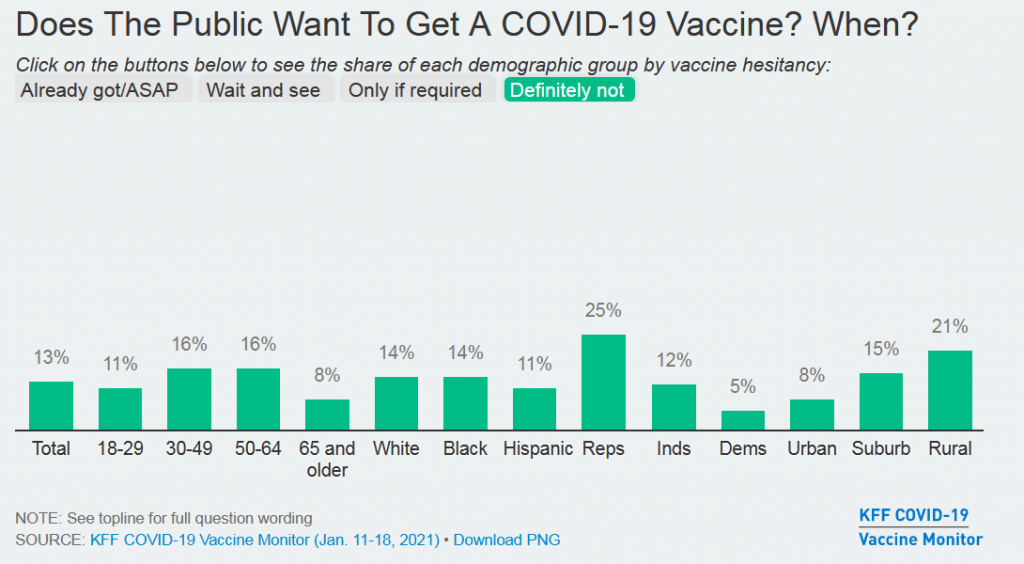
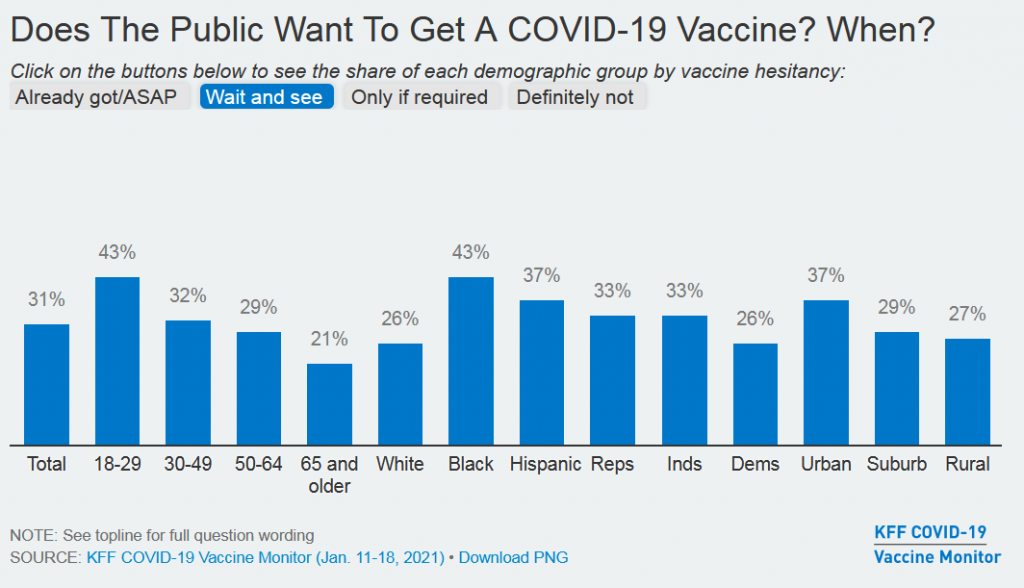
Get In-depth Biotech Coverage with Timmerman Report.
22
Feb
2021
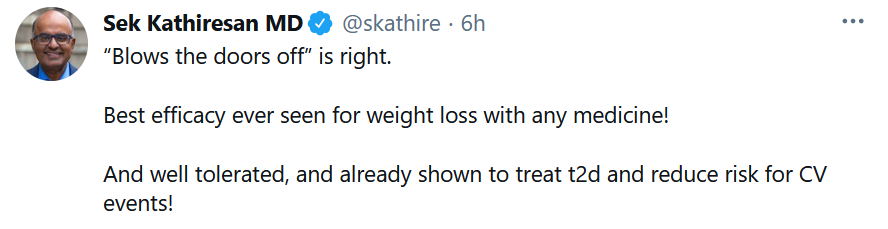
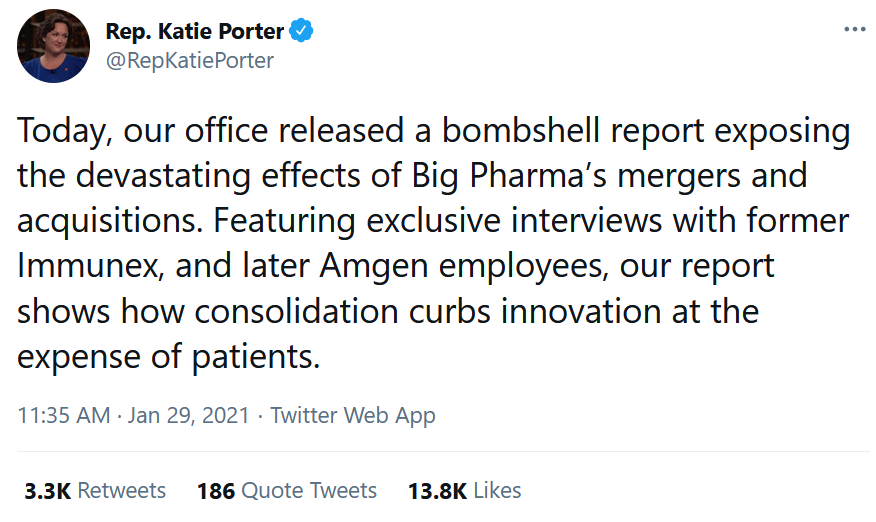
Big News for Obesity and Heart Failure That’s Been Underappreciated
Please subscribe and tell your friends why it’s worthwhile. Quality journalism costs money. When you subscribe to Timmerman Report at $169 per year, you reward quality independent biotech reporting, and encourage more.