Get In-depth Biotech Coverage with Timmerman Report.
24
Nov
2020
Getting Married in 2020. One of Many Difficult Family Decisions

Carolyn Ng, managing director, Vertex Ventures HC
So there it was, hanging in the closet.
My carefully chosen bridal gown, with its lace in a delicate shade of ivory. Beside it was my bridal veil, featherlight, translucent, and freshly purchased just the day before the wedding.
When Anna, my girlfriend, gingerly pinned it on my head, I heard the words of my mother sounding in my head. She had once told me, sweet and soft, “Don’t forget your veil. There is nothing more bridal and more beautiful than a bridal veil.”
My heart sank for a moment. Because I know my mother would have given anything to see me walk down the aisle as a bride, veil and all.
Yet I had to tell her, no, mum, Matt and I are getting married, but you will not be able to attend in person. We won’t allow you, or other family members to attend.
It was a difficult conversation, and 2020 was full of all the most difficult conversations, for us as individuals, as a family, as a country.
As the years pass, and as Matt and I grow old together, what would we want our wedding day to be remembered for? I want it to be centered on us and our love. But for it to be about us and our love, it has to, by my definition, naturally include all the important people around us in our lives. Friends and family who have shaped us, challenged us, cared for us, and molded Matt and I to be who we are today, choosing each other as life partners.
There is no celebration of “us,” if it does not include “them.”
And that is why this COVID-19 pandemic has taken such a toll on us all. It is not only the headlines-grabbing devastation that has robbed the US of more than 250,000 lives and over 20 million jobs. What is also draining and painful is the multitude of mundane daily decisions we have all been deliberating over and over in the past nine months, calculating the risks of a deadly virus exposure against the very human need for togetherness.
After all, it is this need for social interactions and physical closeness that define us all as human beings.
Weddings present a special conundrum in the pandemic. We got engaged in November 2019, and were originally planning to be married in July of 2020.
How did we change plans? Well, one thing for sure, we certainly did NOT want our parents and aunts and uncles and any of our lovely guests catching the SARS-CoV-2 virus from our wedding. Given our professions — we are both healthcare venture capitalists — we have front row access to scientific and clinical knowledge pertaining to the SARS-CoV-2 virus and what it is doing to public health and the economy.
They say with knowledge comes power. And I would add, the power to act responsibly.
So, some tears were shed. Some hearts broken.
But eventually all of our parents and family members agreed to stay in the safety of their respective homes and not fly to attend our wedding in person. Instead of the original 150-person guest list, we had a couple of our dearest local friends witness (and operate the Zoom for) our wedding, which they did with aplomb. Our wedding was held outdoors in a beautiful private garden, and we also made sure that all of us involved had a negative COVID-19 test within that week of our wedding. Face masks were given to our in-person guests as wedding favors.
It was not completely risk-free. We recognized that. But we could conscientiously say that utmost effort was taken to reduce COVID-19 risk to as low as possible, while allowing for a figment of magic to still be conjured for our special day.
I would like to think there was still magic in the air, and that all of our Zoom wedding guests could feel it. It was as magical as a video technology like Zoom could allow you to feel. We had a couple of technical glitches of course, as any respectable wedding would have. But save for a few hangovers, we were immensely fortunate that our beautiful wedding concluded with no real sequelae. I mean the COVID-19 types.
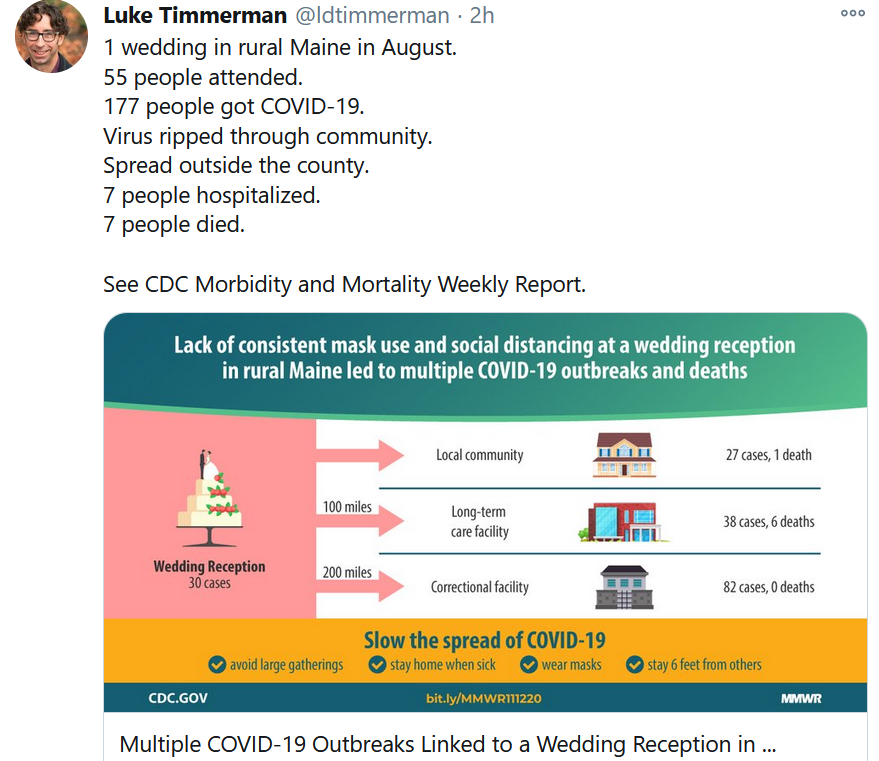
Not every wedding couple has been as lucky as we were. Some took bigger risks and faced dire and deadly consequences. Barely two weeks after our nuptials, I read with a heavy heart an article authored by the CDC on the wedding in August which turned into a nightmarish super-spreader event.
In that case, the couple from California held a 55-person indoor wedding in rural Maine. It was reported that almost none of the guests wore masks indoors, nor did they maintain physical distancing of at least 6 feet apart. At least 177 infection cases were linked to this single event, including outbreaks at a long-term care facility and a correctional facility, which were 100 and 200 miles away from the wedding venue!
Tragically, seven people died from COVID-19 as a result of this wedding.
And even more tragically, perhaps, none of those who died were even in attendance. They were all secondary and tertiary infection cases. The virus has won the R0 game.
To have your wedding be the cause of seven innocent people’s deaths? I cannot imagine the pain and anguish of the newlywed couple. That burden of guilt and despair that will forever lace the memory of the day they tied the knot. An event of negligence, a lifetime of regrets.
Regrets. We desperately try to avoid them. Every critical decision, step, action that we make in our lives, we are propelled to take the ones that will render the highest probability of “no regrets”.
In a pandemic, these decisions that would give us “no regrets” become way more difficult to make.
Pre-COVID-19, we had been talking fondly, for the longest time, of a visit to Kentucky. There, we would visit Matt’s grandmother. He calls her “Mommom” in the most endearing way, and I hear lovely stories of how he grew up spending the most wonderful time with her when she was still living in Philadelphia.
And then the pandemic hit and turned the world upside down, killing people and infecting many more. We put our Kentucky trip plans on ice. We could visit her in 2021, we said. Vaccines would be coming and we would be visiting her safely, we said. We would also do a bourbon tasting tour, we said.
Mommom will love you so much when she meets you, Matt said.
Mommom, 91, passed away this past weekend.
We never made it out to Kentucky. Matt did not get to say his good-bye to his closest grandmother. I never got to meet this incredible woman who was part of what made Matt who he is today.
Regrets and also sorrow. How desperately we avoid them. Our scientist/doctor selves keep reminding us that it was a prudent decision we made not flying over this year and introducing additional virus risk to Mommom who was obviously in a high-risk age group. But nothing can stop our human hearts from bleeding.
We can’t help but ask ourselves, “Could we have done this?” or “Could we have done that?” to see her one last time?
We felt the tug again to see family this Thanksgiving. But seeing the surge to more than 180,000 cases a day in the US, we decided to cancel our flights from San Francisco to Philadelphia. It is cold this time of the year in Philly, rendering outdoor dining impractical and social distancing difficult. Flying also introduces additional risks to our parents and we are not confident how we can mitigate them without quarantines and testing. In short, we will not be tasting Matt’s mum’s famous 25-pound turkey this year.
So with Thanksgiving and Christmas around the corner, how would you like this year’s special family holidays to be remembered?
There are trade-offs in every arrangement we make in the midst of a global pandemic. But may we choose actions that we may live with for years to come even post-pandemic, conscientiously, responsibly and lovingly. And without regrets, if we are so blessed.

Carolyn Ng and Matt McAviney at their wedding. (Credit: iPhone photography by Kevin Dai, Vivo Capital)
Written, in loving tribute, to my husband Matt McAviney’s grandmother “Mommom”, who left us this past weekend peacefully. I wish I have had the chance to get to know you.