Get In-depth Biotech Coverage with Timmerman Report.
19
Jul
2021
From Delta…to Omega?

Otello Stampacchia, founder, Omega Funds (illustration by Praveen Tipirneni)
I hope you are all enjoying your summer break with (finally) being able to see friends and family (and fine food NOT from a delivery service).
You are? Great!
Now let me spoil some of that for you.
- This pandemic is not over
I understand the inclination to consider the pandemic behind us. In large parts of the US / North America and in (most of) Western Europe, vaccines (largely the very effective mRNA kind, with the UK being a notable exception) have been rolled out quite effectively and have now been administered to large percentages of the elderly and of the “at risk” population.
Cases and deaths have been decreasing for months in the same locations and are now a fraction of the (horrific) numbers we had last winter.
However, the (now infamous) Delta variant (originally discovered in India) is spreading at accelerating rates across the world. This includes (worryingly) countries who previously faced other variants of concern (VoCs), such as Alpha (discovered in the UK) and Beta (discovered in South Africa): it is particularly concerning to notice the exponential spread of Delta in the UK, a country that is now in its summer, had already been ravaged by Alpha last winter, and has fully vaccinated most of its vulnerable population with the adenoviral vector-based AstraZeneca vaccine.
I do understand the impulse to rush to Wembley to watch the home team lose to the Italians, but still. Similarly, South Africa has been facing an exponential growth in cases since June.
I can already hear you: “The increase in the number of cases is not resulting in an increase in hospitalizations and deaths. That link has been broken!”
First of all, and not to repeat myself: increases in cases appear earlier than increases in hospitalizations, which themselves lag behind an increase in deaths, each by 2-3 weeks. We already are noticing meaningful increases in hospitalizations amongst the unvaccinated / younger populations in the UK and US.
It is also true that the young (<50 years old) are at lower (relative) risk of hospitalization and severe disease. That said, the absolute number of people who will now potentially be infected is higher since Delta is super contagious AND vaccination rates are low amongst the young.
Second of all:
- it is TRUE that doubly-vaccinated, “healthy” people are strongly protected from severe symptoms and hospitalizations (even from Delta infections); that said, and as eloquently said by Larry Corey in this June 2021 Timmerman Report, there are UP TO 15 MILLION PEOPLE, in the US alone, who are immunocompromised / immunodeficient. These are cancer / HIV / transplant patients, amongst others. Vaccines have not been tested in clinical studies in these populations. There is now a large body of evidence that antibody responses in these groups wanes fairly quickly even after double vaccination. T cell response might last longer than antibody levels and might confer some protection, but we simply do not know how much or for how long: it remains unclear the amount of protection even mRNA vaccines might be able to provide, in the long run, to these patients;
- Even in the US and Western Europe, there are significant pockets of vaccine-deniers: refusing to be vaccinated is already resulting (in the US especially) in significant increases in hospitalizations in certain locations. This fall we will have also to contend (again) with more indoor transmission and the re-emergence of other transmissible viruses (flu, RSV, etc.) (FYI: I am NEVER shaking another person’s hand again.
I doubt we will see a repeat of the horrific statistics we saw last winter in the US / Western Europe (vaccination levels were almost nil back then).
However, even now, the US is tracking just below ~300 deaths / day from the pandemic, almost entirely in unvaccinated individuals. And we are in the summer, where opportunity for super-spreader events are much more limited. Without a new surge in vaccinations, and with the Delta variant in wide circulation, we could very well see a corresponding increase in deaths this fall when more people are gathering indoors.
Now, I know this is not a very popular point of view, but let’s try to forget American exceptionalism (for just a minute) and look at what is happening in other countries who do not have the luxury of having access to efficacious vaccines for their population.
Across the world, and in particular in South America, Southeast Asia, and Africa, the conditions are horrific. A lethal combination of insufficient vaccination levels (because of lack of supply), variants spreading (Delta above all, with Lambda being a possible concern in South America) and winter kicking in in the Southern hemisphere, is wreaking havoc on these countries.
Even countries who successfully contained the virus early on (Australia, Japan, Indonesia, Vietnam, etc.) are being forced into repeated lock-downs because of the lack of vaccine supplies and the spread of the Delta variant. South Africa has <3% of its population fully vaccinated, Indonesia <6%: both countries currently report record rises in cases, at levels that are multiple times higher than last January.
- The increasing risk of more variants of concern
As I mentioned in February, (I am NOT going to change my already tragic first name to Cassandra (thank you very much!): there are no precedents in humanity’s history for a pandemic confronted in similar conditions: 8 billion susceptible individuals, many with co-morbidities (see below), and relatively unfettered, almost instantaneous travel between countries. The Delta variant was sequenced in Dec 2020, and it has since become the most prevalent variant across the world.
Its evolutionary fitness is something to behold. One study earlier in July from Guangdong found that viral loads in patients with the Delta variant were ~1000 times higher than with previous variants on the day the disease was first detected. It also appears people shed viral particles much earlier when infected with Delta. No wonder we’re seeing Delta spread so remarkably fast.
The one, I believe unprecedented factor that absolutely terrifies me, however, is the almost ideal incubation factory for novel, fitter viruses represented by immunocompromised / immunodeficient individuals.
One case from June might highlight this concern: a 36-year-old woman with HIV from South Africa was diagnosed with COVID and followed by her physicians. The virus was found to persist in her (immunodeficient / immunocompromised) body for over 200 days and to accumulate 32 mutations during this period, 13 to the spike protein and 19 others across the viral genome.
That’s a lot of opportunity, in just one individual, for SARS-CoV-2 to find ways to evade the host’s immune reaction. Now consider how many individuals in modern societies are immunocompromised. The virus has a mind-boggling number of opportunities to develop escape mutations, and it’s moving through large populations at exponential speed.
The only long-term solution for developing countries is to vaccinate their populations as soon as possible: they do not have the luxury of prolonged lock-downs since their governments will not be able to provide the same level of support that Western governments enabled for their workers and economies. Therefore, for the time being, the pandemic will continue to ravage Africa, South America and Southeast Asia with relative impunity.
It is anybody’s guess how long it will take to vaccinate developing countries. We’ve already seen India — the major producer of AstraZeneca’s vaccine for the COVAX facility — halt some of its vaccine exports to better vaccinate its own vulnerable populations. India reportedly made this decision in the aftermath of its tragic Delta-fueled surge this past spring.
A wild guess, but I am thinking it will probably take an additional year / 18 months or so to cover the world with vaccines. And this could be an optimistic scenario, as it assumes a) Western countries avoid falling into more “vaccine nationalism”, as their own populations claim booster shots and b) ALSO that manufacturing has been scaled up.
Add to this timeline the extremely worrying fact that we are still not performing enough sequencing of potential emerging variants (including, and criminally so, in the US), and we might be blindsided again this winter from another variant which might be as infectious as Delta and perhaps better at evading the immune system than Gamma or Beta. I am not sure what the probability of that is. It is far from zero.
By the way, the potential long-term geopolitical repercussions of letting the pandemic ravage uncontrolled in these countries could be absolutely devastating to the current world order. Now, if you want to chat about that, let me know, but I do not want to depress readers any further.
- Some predictions
I am sure you are, by now, sick of hearing me prophesizing doom and gloom every time that you think you can finally go out on vacation and meet friends. Therefore, before starting my own little break, I am providing below a table describing what might be the short-medium term outlook in different parts of the world.
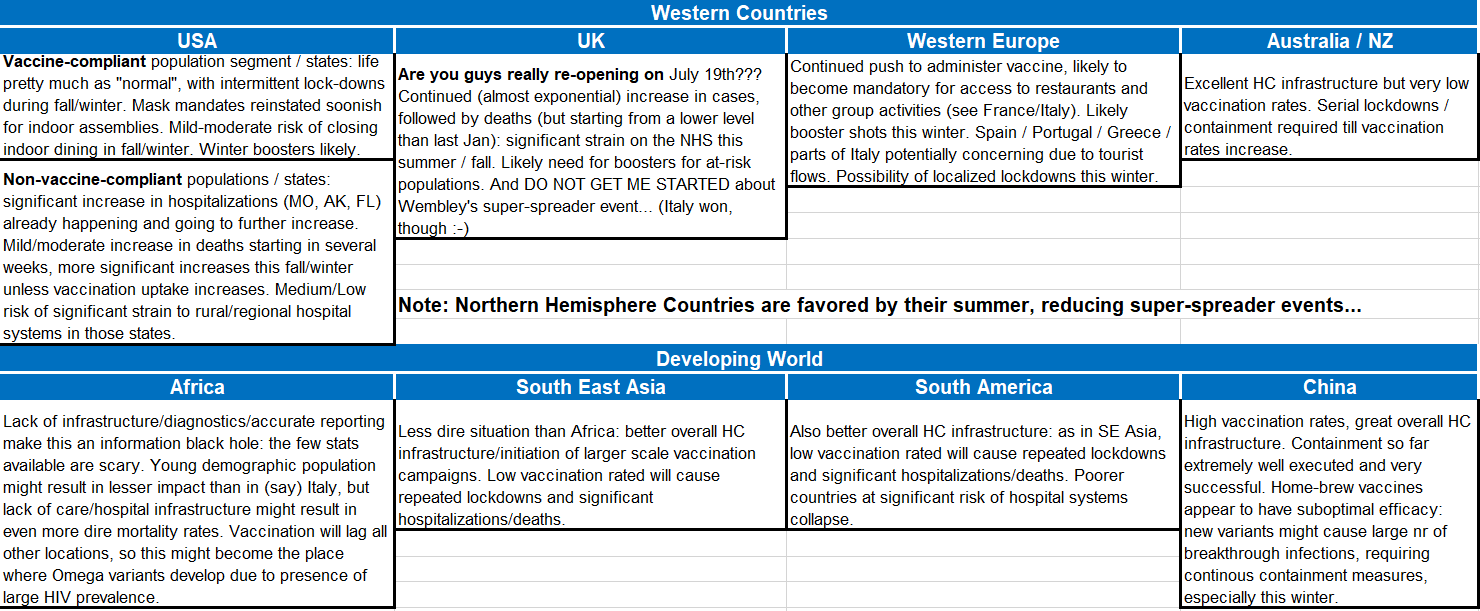
All this, without being able (of course) to assess the consequences of an “Omega” variant that resists / escapes vaccine-induced immunity. If such a variant emerges, then, as I wrote in my first Timmerman Report on Mar. 10, 2020:
“May the fates look upon us with mercy”.
Follow Otello Stampacchia on Twitter: @OtelloVC
This article expresses the personal views and perspectives of the author. The views and perspectives expressed here do not necessarily represent the views or perspectives of Omega Fund Management, LLC or any officer, director, partner, member, manager or employee of Omega Fund Management, LLC or any of its affiliated entities.





















































